| Pages:
1
..
21
22
23
24
25
26 |
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I like how easy is to replicate "complicated" industrial processes by some glass, kanthal and copper wire
When you isolate the aldehyde, be careful to fully avoid the alcohol carry over into the bisulphite solution since it would precipitate out the
bisulphite before the adduct can form, as your experiment pointed out in the acetaldehyde thread.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, we should do more of this. But you did hire a glassblower, right?
Quote: Originally posted by Jimmymajesty  |
When you isolate the aldehyde, be careful to fully avoid the alcohol carry over into the bisulphite solution since it would precipitate out the
bisulphite before the adduct can form, as your experiment pointed out in the acetaldehyde thread. |
The bisulfite is added as a saturated aqueous solution. Since this is biphasic with the aldehyde I had to shake it vigorously for 1/2 hour (per
procedure) to get the adduct to form. This appeared to work well.
[Edited on 29-3-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Magpie, is hiring a glassblower really such big a deal? In every town there are at least two, they always complaining not to have enough work so the
one I usually go to quite happy when he sees me.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
No, hiring a glassblower is not a big deal and I encourage it. I have hired glassblowers to make borosilicate and quartz labware.
But finding one locally was not possible for me. I tried two. Neither would do work for private citizens, only business or institutions. They were
concerned that the police would think they were helping drug cooks. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
IMHO it is not polite to ask someone why do you need this and that. I mean what is the point asking it? It is like being asked by the bank, why do you
need money? You do not wana do sumthin illegal do you?
Glassblowers with this attitute better be avoided and I am sad that cooks made things so hairy in your town.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi Folks,
I did another 2 hrs run in the weekend but this time, -seeing Magpie's salicyl aldehyde post- I isolated the benzaldehyde.
Started off with 328g Toluene, it was oxidized over copper wire for 2 hours.
Then 60g potassium metabisulphite was added. - nothing precipitated
The bisulphite was "neutralized" with 60g sodium carbonate (the solution became turbid + benzaldehyde smell)
Fractionated some gasoline: 60-75°C fraction was collected. This was used to extract the benzaldehyde ~0,5l
The C5-6 was distilled off on a water bath then I let air flow through the flask for 1 min to remove any traces of solvent.
The remaining liquid - benzaldehyde - was 1g
I let butane into the vial to inhibit the oxidation then capped.
Considering that after the bisulphite addition I got back 312g toluene so a total of 16g toluene was oxidized it is not so bad. I mean I can let the
setup run for the whole weekend. That would yield 24g 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's a slow production rate - but you have proved that it works in principle.
Did you shake the benzaldehyde/bisulfite mix for a long time? The mix is biphasic so the violent prolonged shaking is necessary.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I know it is slow and it is only a proof that it may be possible to make decent amount of bzaldehyde by means of vapor phase oxidation. I think I have
just made the most expensive 1g of benzaldehyde of all times, still I would call it a success.
Yes I shook it with bisulfite for 30min, then I shook the toluene phase with water to check for benzaldehyde smell and the result was negative. I
think I lost some however during the extraction with alkanes. After shaking 100ml benzaldehyde+water with 100ml alkane the benzaldehyde smell was
persent in the water phase every time.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi again,
Now that the weekend is coming and I am antisocial enough to spend the time in the garage rather than outside I will try out the oxidation with CuO.
The copper oxide was prepared by anodic oxidation, the mica support with the kanthal wire was immersed into the CuO paste and dried with the filament,
this was repeated couple of times. Of course the CuO is very porous so large chunks fell from the support during the assembling. It may all fall off
when it gets into contact with toluene... we will see.
Anyway I think this as much chance as the benzaldehyde can get to form with this setup, so if I can not make more than last time I will not try it
anymore.
  
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very nice! As I've said before you are doing such original work: a real pioneer.
I have to ask: Was that element salvaged from a toaster? It sure looks like it.
I just happen to be working on a project where I might use the toaster element as a heater as it has a high specific resistance. I have measured it
as 2.5Ω/10cm.
-------------------------------------------
Fresh results from the lab:
at 4a, R=2Ω/10cm; for 2a, R=1.7Ω/10cm
At 4a the wire is orange red, like in the toaster.
At 2a the wire has not changed color.
[Edited on 9-4-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Thanks Magpie,
It was not actually a toaster but your guess was very close it was in some hotplate.
What do you want to heat/pyrolyse with it?
So I did a run with CuO. As soon as I cranked up the power on the filament all the CuO turned to Cu in an instant. I wanted to operate under a mild
condition, so I turned up slowly the wattage when the filament became incandescent I turned it down a bit.
Also I set the air load to such level that no Benzoic acid fumes were apparent at the outlet of the setup.
These setting yielded nothing... after 2 hours I took a sample from the toluene shook it with water, there was not any benzaldehyde in it.
So I think the benzoic acid vapors and the yellowing of the toluene indicates that the oxidation is going, without these you only wasting time.
This setup was not made to do this oxidation specifically, so I will come back with the good old horizontal reactor layout and see if I can make any
uselful amount.
I have many improvements in mind, so wait for it!
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Reaxys search of all possible way for making Benzaldehyde from Toluene
Attachment: Toluene to Benzaldehyde.pdf (1.2MB)
This file has been downloaded 2867 times
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I'm interested in performing the synthesis of o-nitrobenzaldehyde from o-nitrotoluene (for indigo synthesis). I've read through parts of this thread,
and looked at some other resources from around the internet, but I am still not sure what the optimal procedure is. It seems like there are a lot of
different ones with many variations and usually rather poor yields. Plus they're optimized for unsubstituted toluene and I don't know how they would
work with the substituted version.
The sulfuric acid/manganese dioxide way sounds easy, but appears to be very unreliable in practice. The electrolytic procedures seem pretty good, but
my electrochem skills are garbage so I don't think I'm going down that path. This one using chromium trioxide in acetic anhydride might actually be feasible if I am allowed to do it at school. Might an Étard reaction work for the substituted toluene? I'd prefer not working with chromyl chloride, but if it works well, I would consider it.
I'm simply not experienced enough to judge which procedure is best for me. I was hoping that someone who is more familiar with the contents of this
thread, or with toluene to benzaldehyde oxidations in general might recommend a procedure that would fit my needs. I would just go and try a few
different methods, but it was quite time consuming making and isolating the o-nitrotoluene, so I'm being very cautious.
Thanks.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The autooxidation of ortho-nitrotoluene, or the oxidation of its carbonanion by oxygen, can give ortho-nitrobenzaldehyde as the
major product under proper conditions (alongside other products). There are numerous examples in the literature, though mostly in Chinese. For
example, already stirring it in DMF at -10 °C can give a 30% yield (DOI: 10.3891/acta.chem.scand.25-3509). Oxidations with oxygen are catalyzed by
transition metals and can give good yields (for example with Co(acac)2, DOI: 10.1055/s-0032-1318172; another one using MnSO4 is
in DOI: 10.1023/A:1025824528577 and EP671381).
Alternatively, many published methods go trough a two step process of condensing with DMFDMA followed by oxidative cleavage to get the aldehyde (one
such example is in DOI: 10.1080/00397911.2010.517366).
The oxidation with MnO2 in aq. H2SO4 is described in DE179589 (from 1899). It does not sound very safe as it is done
in an autoclave reactor.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Ah, thank you Nicodem. I think I'll look into the manganese sulfate catalyzed one. Time to go see if it's on SciHub. 
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Controlled oxidation of alkyl chain of Toluenes (substituted or otherwise) to aldehyde by MnO2 in presence of H2SO4 is an extremely tricky process.
Many old literature references talk about it in a very casual way as if the MnO2 is a pure reagent. In fact MnO2 being an ore has got extremely
complex and complicated composition, crystalline structure and activity. "Unit Processes in Organic Synthesis by Groggins" describes it as a method of
making benzaldehyde from toluene from old German reference. IIRC, the temperature is 40 Dec C and conversion stated is 20 %. When I attempted this
reaction with local MnO2 ore (Manganese Ore India Ltd) having 80% MnO2, I got nothing. When heated to the reflux temperature, I got Benzoic Acid and
no Benzaldehyde.
Substituted toluenes fare batter than toluene. I know of a company in India which makes Anisaldehyde (p-methoxy benzaldehyde) by MnO2 + H2SO4
oxidation route. They are one of the largest manufacturers of Anisaldehyde in the world and as a by product produce huge quantity of MnSO4 powder.
p-Cresol --> p-Methoxy Toluene --> Anisaldehyde
But the process is extremely delicate. They say that the MnO2 ore from only one particular mine does give them proper yields and a product free of
impurity (of p-methoxy benzoic acid).
The synthetic MnO2s (EMD and CMD) - besides being very costly - are highly reactive and give benzoic acids rather than aldehydes.
Numerous methods in literature are given to "activate" an otherwise inert MnO2 ore. Some of them are very simple such as treating the ore with dilute
Hydrochloric or Nitric Acid, but the results are still highly unpredictable and processes have to be validated on case to case basis. It is an ideal
job for process development chemist.
gsd
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
gsd, you are overemphasizing the problem. If you would try to use e.g. dead burnt ZnO as a catalyst for diol-urea esterification, you would obviously
get 0% yield, because this kind of zinc oxide is almost completely unreactive. Iron-acid can reduce aldehyde to alcohol, but if you drop a nail into
acid-aldehyde mixture, you will get no reaction. Just like manganese oxide ore is unreactive towards almost anything.
Do you know that polyethylene terephthalate is actually stable towards dillute hydrochloric or sulfuric acid? Despite the fact PET is an ester and
should be hydrolyzed by acid.
If you drop a stone of calcite into an acid, it will take hours or even days to dissolve it, depending on the source and size of the stone.
There's 23 pages of discussion, I'm sure someone have already mentioned that you cannot use a regular MnO2 ore for toluene oxidation.
Benzaldehyde is one of the strongest oxidizers among the aldehydes, as well as they are good oxidation catalysts. To further oxidize benzaldehyde into
benzoic acid, you need to fully exhaust the toluene-benzyl alcohol reagents.
Synthetic active MnO2 is easy and cheap to make, as well as the results of its application is predictable.
UPD: I've realized methylbenzene oxidation is not the same as a regular benzyl alcohol oxidation. Thus, MnO2 for the oxidation works or industrial
production, but it's pretty much useless for laboratory preparative use. Here's the reason why: γ-MnO2 octahedral molecular sieve: Preparation, characterization, and catalytic activity in the atmospheric oxidation of toluene
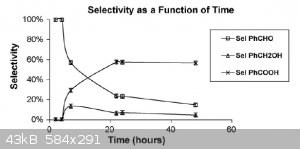
As you can see, once you have benaldehyde in the reaction - it starts converting into benzoic acid, despite the fact that toluene is oxidized into
benzaldehyde with very high selectivity.
I would recomment using of permanganate or some other reagent more selective reagent for the task. A Convenient Oxidation of Benzylic Methyl, Methylene, and Methine Groups with Potassium Permanganate/Triethylamine Reagent
[Edited on 14-1-2016 by byko3y]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by gsd  | | The synthetic MnO2s (EMD and CMD) - besides being very costly - are highly reactive and give benzoic acids rather than aldehydes.
|
Molinari specifies CMD as does Compt. rend. 634 (1901). I suspect the technique has a bit to do with it.
PS: And also IIRC in the relevant patents DE101221, DE107722, and FR276258.
PS2: US613460 indicates a CMD of vague hydration; there is no activation step: "Up to the present I have obtained the best results by the use of
freshly-precipitated manganese binoxid or Weldon mud and sulfuric acid as the oxidizing agent"
[Edited on 15-1-2016 by S.C. Wack]
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
@byko3y here you are confusing between MnO2 as Catalyst and MnO2 as reagent.
The ref you have quoted uses γ-MnO2 as a catalyst for oxidation of toluene with molecular oxygen. To quote from the abstract that reference:
"A synthesized γ-MnO2 octahedral molecular sieve was characterized and used to catalyze solvent-free atmospheric oxidation of toluene with molecular
oxygen. The γ-MnO2 showed excellent catalytic activity and good selectivity under the mild atmospheric reflux system at a low temperature (110 °C).
Under optimized conditions, a 47.8% conversion of toluene, along with 57% selectivity of benzoic acid and 15% of benzaldehyde were obtained."
(BTW with 57% vs 15% selectivity in favor of benzoic acid, this is a process for acid with aldehyde as a welcome by-product)
I was discussing and commenting on the use of MnO2 as a reagent.
KMnO4 is a different ball game altogether. It is like American Football to me which I have never played.
gsd
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Thanks SCW for the patents ref.
This is the patent referred in the Groggins book.
gsd
Attachment: DE101221C.pdf (185kB)
This file has been downloaded 856 times
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi there,
So my horizontal oxidation reactor seems to be complete.
Features:
Wattage controlled reboiler and preheating zone.
Good old -kanthal wire wound around Quartz tube- reactor.
Copper wire catalyst with very low residence time.
Glass thermowell to save the PTFE tape seal.
Air as oxidant
So I filled her up with ~0,5l previously pyrolysed toluene. The plan is to run it for at least 8 hours.
I am wondering how high can I go up to with temp at the preheating zone without damaging the PTFE tape seal at the end of the reactor. This can limit
the oxidation. I have to go up to 400°C at least or I am just wasting my time.
Actually the plan is to see some incandescene at the copper then turn the heating down a lil bit till I see no light in the dark, then It should
produce benzaldehyde 1/3 and benzoic acid 2/3.
Lot of questions here but they will all be answered in the coming days
![WP_20160129_22_18_17_Pro[1].jpg - 1.9MB](https://www.sciencemadness.org/whisper/files.php?pid=437090&aid=47698) ![WP_20160129_22_18_36_Pro[1].jpg - 1.7MB](https://www.sciencemadness.org/whisper/files.php?pid=437090&aid=47700) ![WP_20160129_22_18_58_Pro[1].jpg - 1.6MB](https://www.sciencemadness.org/whisper/files.php?pid=437090&aid=47702)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's interesting work Jimmy. Can you give us some words of explanation to go with the pictures.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Is there a way to de-energize the oxidation reax? 25% yield is not particularly auspicious. OAS: purification of benzal usually involves washing first
with NaOH or Na2CO3 followed by bisulfite (aq) and distillation under aspirator vacuum. IMHO if you're pulling a vacuum N2 atmosphere can be
neglected. @Jimmy: In the picture above left, the heating mantle is not sized for the flask and you might burn it up unless you pack foil around the
flask. I like your inventiveness, reminds me of some of the stuff Rappaport used to show us.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hear, hear!
I'm particularly interested in the tubular reactor: wattage and type of resistor.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
So... Toluene boils and it goes up in 1m long glass tube, at half way up is a thermometer to monitor the vapor temp, this long distance may prevent
benzaldehyde carryover since it would be oxidized to benzoic acid or pyrolysed into benzene if it would hit the copper again. At the top of the column
(at the inlet of the quartz tube) is the air inlet, air is supplied by an aqua pump.
The reactor is 1m long quartz tube the copper wire is at half way, before this is some raschig to help toluene mixing with air.
There is a grounded glass joint insert at both end of the reactor that were accomodated and sealed by PTFE tape, that is the reason why i have to
monitor the temp at the end.
The toluene boils somewhat separates from the heavies on its way up, then it mixes with air, preheats by means of kanthal wire, hits the catalyst,
cools somewhat, then completely condenses in the condenser, flows back into the 2l RBFL, so basically the content of the RBFL is never gets into
contact with air.
So the 8hr run has started about 1 hr ago, the toluene boils so there is 3drop/sec at the condenser (may be too high) the temp at the end of the
reactor is 250°C. I am a bit skeptical that I will be able to recover any useful amount of Benzaldehyde, but who knows, the copper is working though
evidenced by the color change at around 230°C. As I understand it, when the CuO loses its O2 and its converted back to Cu or Cu2O indicated by the
color change means the copper started to act as a hydrogenation or oxidation catalyst. In this reducing atmosphere Cu2O oxidizes the toluene with one
molecule oxygen and it keeps being regenerated by molecular O2 from air.
There are benzoic acid crystals on the wall of the reactor after the catalyst so it kinda works.
The wattage of the kanthal on the reactor is 1500W.
Yeah I often char things... mainly on purpose thanks for the advice though! thanks for the advice though!
![IMG_3845[1].JPG - 451kB](https://www.sciencemadness.org/whisper/files.php?pid=437165&aid=47710)
[Edited on 30-1-2016 by Jimmymajesty]
[Edited on 30-1-2016 by Jimmymajesty]
[Edited on 30-1-2016 by Jimmymajesty]
|
|
|
| Pages:
1
..
21
22
23
24
25
26 |