| Pages:
1
..
21
22
23
24
25
..
28 |
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
When pouring liquid Phtalimide after reacting Phtalic Anyhdride / Ammonia into a dish does anyone have a trick for preventing the fine light silvery
dust diffusing into the air around? It solidifies immediately on contact with a room temperature container. The last time i attempted this my product
was off-white, i'm wondering if there was ammonia residue left.
|
|
|
Formula409
Hazard to Others
  
Posts: 129
Registered: 13-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by sparkgap
Apparently; your "n", if it does imply that it's attached to the nitrogen atom should be capitalized. Anyway, how about drawing the structure so we
all have a better idea of what to look for?
sparky (~_~) |
Wiki says that Camphor is 1,7,7-trimethylbicyclo [2.2.1]heptan-2-one, so I am assuming that the methyl amine of it is 1,7,7-trimethylbicyclo
[2.2.1]heptan-2-N-methylamine?

Formula409.
[Edited on 26-2-2009 by Formula409]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Which isomer? Endo or exo at the amine group? Why don't you give a CAS number or correct name. It is pointless to search for information about a
compound only to latter find that I wasted time with the wrong one.
|
|
|
Formula409
Hazard to Others
  
Posts: 129
Registered: 13-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nicodem
Which isomer? Endo or exo at the amine group? |
d-Camphor and Exo at the amine please.
Formula409.
[Edited on 26-2-2009 by Formula409]
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Methylamination of camphor gives d,l N methylbornylamine. The isomeric bornylamines melt at 163C and 180C and boil at 189C and 199C respectively, so
you can expect the N methyl values to be even higher.
It would be typical of hydrocarbon amines in that it would dissolve in organic solvents, would be insoluble in H2O and would form H2O-soluble
hydrochloride salts.
[Edited on 26-2-2009 by manimal]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
(1R)-N-Methylisobornylamin: bp 89-90°C at 10 torr; Alpha<sub>D</sub> = -73.2° (EtOH, 20-23°C)
Derivatives: hydrochloride mp >320°C, Alpha<sub>D</sub> = -59.7° (EtOH); hydroiodide mp 203-205°C; picrate mp 171.5-172.5°C.
Reference: Collection of Czechoslovak Chemical Communications, 26 (1961) 2602-2605
(1R)-N-Methylbornylamin: bp 205°C at 759 torr, 95-110°C at 22 torr, 82-83°C at 10 torr; Alpha<sub>D</sub> = 77.3°C
(EtOH, 20°C)
Derivatives: hydrochloride mp 338-339°C; picrate mp 178-179°C, 178.5-179°C
Reference: Collection of Czechoslovak Chemical Communications, 26 (1961) 2602-2605;
Journal of the Chemical Society, 75 (1899) 1152-1153;
Bulletin of the Chemical Society of Japan, 49 (1976) 1897-1898.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I had a biology lesson today in which we were testing for sugars and starch with iodine (+KI) and benedicts solution.
The results of the tests, as im sure many of you know, is the benedicts forms a Cu2O precipitate in the presence of a reducing sugar, and the iodine
solution goes deep blue in the presence of starch.
After completing a test for sugar with benedicts and getting the Cu2O precipitate I was curious so I added some iodine solution to this precipitated
mix.
The results were unexpected and even my teacher couldn’t explain it- At first the blue color appeared showing starch was indeed present, and masking
the color of the Cu2O at the same time. After a few seconds the deep blue color slowly faded and turned back to the normal Cu2O color. This can be
repeated many times.
Can somebody explain what is going on here? Is the Cu2O slowly reducing the I2 complex?
Thanks in advance,
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Was the Benedicts solution (blue) reduced fully to the orange/red cuprous oxide? What sugar were you testing and was it pure? Also Benedicts solution
is a complex organic using citrate complexed-Cu2+, repeat the experiment using Fehlings solution and add the iodine again. Also you could just test to
see what is observed when I2 is added to neat Benedicts, to a suspension of pure Cu2O, and to a solution of the sugar.
My guess is that excess Cu2+ reacted with the I2 and you precipitated white CuI admixed with the orange Cu2O, the blue colour may have been due to
excess Cu2+ contrasting with the white CuI.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I am not sure as to what the sugar was, we were basically given 4 beaker with unknown contents of proteins, sugars and starches. The solution I am
referring to in this did contain starch.
I am not sure if the benedicts had been fully reduced however I am pretty sure it had, it was completely orange in color.
I have doubts as to whether CuI is formed because the solution would of become noticeably lighter with the white ppt. but it didn’t.
Also why the time delay between dark blue (I2/Starch) back to the orange/red ?
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
I'm a little confused by what you are saying.
Let me see if my understanding is correct:
You had 4 beakers of unknowns, the one which tested +ve for reducing sugar, you then added iodine to AFTER you had added and reacted Benedicts. So you
basically had a tube with a bit of sugar solution, Benedicts solution, copper (I) oxide and then added some Iodine solution and you saw a blue colour
followed by a chaneg back to orange?
Well in my experience you tend to use excess Benedicts, so you will likely still have Cu2+, only way to be sure is to allow the Cu2O to ppt and settle
and look at the colour of the supernatent, also you could add excess sugar.
It could be that the solution of sugar was contaminated with some starch, since the starch-iodide is a sensitive test.
Certainly if you started with starch and hydrolysed it to glucose then rana Benedicts then added iodine I wouldn't be at all surprised if you got +ve
result, but that doesn't sound like what happen.
I suggest repeating it in a test tube changing the variables as in my first post.
Also just remembered that Benedicts solution is alkaline IIRC so you will be removing I2 as IO- and I- you likely have a complex set of redox
reactions occuring.
[Edited on 27-2-2009 by panziandi]
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
The starch is colored by I2 via the formation of via I3- anion, that is favorable to occupy some special vacant place in the starch. So if there is no
I3-, the colour should dissapear. I3- is formed by an equilibrium reaction I2 + I- = I3-, and it can break down as well if there is some good I-
consumer (like Cu(+) that forms CuI, Cu2+ that can (not for sure) oxydise I- to give I2). So probably, first starch was coloured with iodine, and then
I3- lost its I- to give an inert CuI(or oxydised by Cu2+), and the colour dissapeared. And due to there is very few I- in comparison to I2(if KI was
not specially added), it took very few Cu+(or Cu2+), thus the experiment can be repeated many times.
All of the written is IMHO
[Edited on 27-2-2009 by Ebao-lu]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
My memory seems to be failing me today... 
So I wanted to ask if there are any halomethylation procedures for aromatic rings that won't destroy ester or acetal groups. I'm pretty sure
concentrated HCl is not nice to those functionalities.
sparky (@_@)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by sparkgap
So I wanted to ask if there are any halomethylation procedures for aromatic rings that won't destroy ester or acetal groups. I'm pretty sure
concentrated HCl is not nice to those functionalities.
sparky (@_@) |
Might start by looking at these
http://pubs.acs.org/doi/abs/10.1021/ja00178a064
http://www3.interscience.wiley.com/journal/114126991/abstrac...
http://www3.interscience.wiley.com/journal/104056097/abstrac...
where the halo-ether is then reacted with the aromatic group using a transition metal halide as catalyst. This might transesterfy, the full articles
might give an answer.
|
|
|
querjek
Hazard to Self
 
Posts: 76
Registered: 26-8-2008
Member Is Offline
Mood: No Mood
|
|
I have a solution with a few mixed quaternary ammonium compounds in water. Is it safe to store this in PETE for a week or two?
it's all about chemistry.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I just seen on How its Made of them using some chemical to etch the stainless steel with a picture of the maker, Any idea of what was used? He put the
chemical on a sponge and just pressed if for a few seconds and it etched the SS. Im thinking acid but im woundering if anyone has a more definitive
answer.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
A mixture of nitric and hydrofluoric acid is typically used in industry...
But this is a rather dangerous acid. Did the user were thick gloves?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by querjek
I have a solution with a few mixed quaternary ammonium compounds in water. Is it safe to store this in PETE for a week or two? |
Depends on concentrations and pH. PETE is a (poly)ester, anything that speeds ester hydrolysis can trash PETE. Polyolefins are safer in most cases.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | Originally posted by Jor
A mixture of nitric and hydrofluoric acid is typically used in industry...
But this is a rather dangerous acid. Did the user were thick gloves? |
You know honestly I cant remember, He was making a sword and had gloves on most of the time so im pretty sure he had them on. He diped a sponge in the
chemicals and pressed it on a stencial and it etched the SS in a split second.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I would never ever store my chemicals in PETE. Not a good plastic for chemicals storage.
I would recommend everyone to store their chemicals in PE (HDPE or LDPE), PP , glass, aluminium (solvents) or PTFE. As far as I know these are about
the only plastics sold as UN-approved containers.
[Edited on 2-3-2009 by Jor]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Will Phosphoric acid reduce with SiO2 and Carbon like calcium phosphate will?
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sedit
Will Phosphoric acid reduce with SiO2 and Carbon like calcium phosphate will? |
Unlikely, unless it is contained in a pressure vessel. Otherwise it will vaporise long before the required temperature is reached.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
I think it may to some degree, but what point would the SiO2 serve? the phosphoric acid certainly isn't going anywhere when it is heated. I'd
imagine that those temperatures carbon would be plenty reactive and a reaction could happen.
It has been noted that when making pyrophosphoric acid from phosphoric acid, the glass gets eaten away slightly.
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Does anyone know about vanillin diethylacetal (or other, maybe cyclic acetal) synthesis? Can it be made from vanillin/alcohol + acidic catalyst(like
with benzaldehyde, piperonal, anisic aldehyde)? Or it is less reactive and requires ethyl ortho-formate?
Second question is wether this acetal is resistant to base or not, and water-containing base particularly? The problem is, that theoretically
phenoxide anion may break down into EtO- and quinoethoxymethide intermediate(under basic conditions), or at normal pH under acid/base catalysis the
acetal can break directly into quinoethoxymethide and EtOH. Or these reactions are unlikely to proceed?
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
And, unless you protect the aldehyde, you will also get the Cannizaro reaction. Can't answer your question, but I'd suggest you get a good book on
protection reactions.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Acetals are hydrolysed by acid only. If you want a carbonyl protecting group thats unreactive towards acid also use a thioacetal. I would use
ethane-1,2-diol or 1-2-dithiol to form the cyclic acetal/thioacetal, as this is general practice as I understand.
Acetal formation is only acid catalysed, so using acid catalyst and an alcohol with a carbonyl will yield the acetal. The whole reaction is in
equilibrium so to make the acetal use a dean stark and toluene solvent, to break the acetal use excess aqueous acid (well lots of water, the acid is
just the catalyst). Making the thioacetal a lewis acid like BF3 is generally used as the catalyst. So best stick to the acetal (use ethane-1,2-diol
and an acid catalyst, I think sulfuric will work fine but p-toluenesulfonic acid would be better soluble with the toluene solvent).
I'm not sure if the acetal you are forming is special or not but I would say it should be unaffected by base just as other acetals are, unless there
are other functionalities present that are base intolerant.
Paddywhacker: You are right that the cannizzaro reaction can occur with basic conditions but the mechanism requires the formation of two negative
charges on the molecule, which is supposedly only going to happen in forcing conditions (conc NaOH, maybe hot also I can't remember).
len1 seemed to find that even a "weak" base like sodium carbonate can cause the reaction come to think of it (see his thread on making benzaldehyde in
prepublication, I think it is via the route of benzyl and benzal chloride).
Edited to add cannizzaro mechanism.
[Edited on 5-3-2009 by DJF90]
[Edited on 5-3-2009 by DJF90]
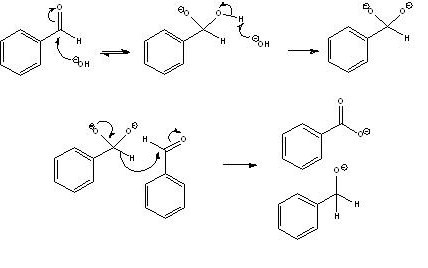
|
|
|
| Pages:
1
..
21
22
23
24
25
..
28 |