| Pages:
1
..
20
21
22
23
24
..
48 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
Have you taken a break 
Edit:
Sorry about that. Your post just appeared. Looks like it's good news all around. End of edit.
Anyhow if perhaps the stuff will (god forbid) not pass current it may have a layer of (shall I say) shit on it.
You have suggested this yourself when you could not weld it first time.
Read below, it is from this book
Page 111
Dann2
[Edited on 9-2-2009 by dann2]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
casting composition for heater tube thermal link
The Pb-Bi eutectic is Pb 44.5% Bi 55.5%, melting at 123.5 °C. Other melting points: Pb 327.5 °C, Bi 271.5 °C. You used to be
able to buy Bismuth shot from shot shell reloading suppliers, but they seem to all have switched to tungsten-based formulations. In any case, some Bi
would lower the melting point of lead enough to avoid worry about frying the insulation. Even so, you'll want to use teflon, which is typically rated
to 200 °C.
You'll want to decide whether to encase the wires or not. Disadvantages are increase material cost (primarily the Bi) and the possibility of shorting
out the leads at their junction with the case of the cartridge. Advantage is lower thermal resistance.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Thank you WF... this is a likely method to both secure the cartridge heater, and ensure that the heat transfers successfully. For a lead dioxide
plating rig, a Titanium heater is second only to an expensvie process heater encased in PTFE, or glass/quartz, in terms of desirability.
I took a careful look at fish heaters, and the hot zone on all of those that were capable of 100 watts + (minimum) were very long, too tall to mount
vertically, and in most cases, too long for a horizontal, submerged system, unless the plating tank is quite large.
Update on the cheap eBay mesh - it continues to conduct, the material is intact, and the products generated (chlorine and hydrogen gas; hypochlorites,
other relatively noxious species) are right in line with previous MMO use for chlorate production. Whatever the stuff is, it appears to be capable.
For a given voltage, current continues to rise, and by extension, when the system is in CC (Constant Current) mode, the voltage for a given amperage
is dropping. Previous examples have shown a similar pattern, with the voltage vs. time curve to be concave... starts high, drops, then begins to
climb as chloride ion concentration decreases.
I will continue to run until I have visible chlorate from this small, 1 liter cell, then pull the plug, and examine the mesh. But as of yet, no signs
of deteriotration. The ONLY issue was the welding problem.
Idea... I'm going to try and do controlled measurements of the resistance of this material vs. the proven mesh. Then, I am going to try an etch in
20% nitric to test both the durability of the oxides, and to see if perhaps there is some chemical residue or more likely some film that inhibits spot
welding. I get the feeling that this MMO is not as electrically conductive as the other material, meaning it'll heat more; BUT, so far, the voltages
have been right in line with previous efforts. Stand by for updates...
[Edited on 9-2-2009 by Swede]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Results of the cheap eBay anode for chlorate production:
Excellent, approaching spectacular. I am amazed at how much was made, so quickly, in such a small cell. I did no calculations, did not measure
chloride, I simply pulled the plug on it when I had to leave town after maybe 2 days.
This was a lowly 1 liter cell, and I started with KCl stock, no used liquor, precursors, or additives of any kind. The current varied between 5 (at
night) to 15 max, at which point the 1 quart plastic bucket began to get too hot.
The bottom of the bucket had about an inch of crystals, which I filtered and washed:
I could see no ill effects on the anode mesh. Color variation in this photo are nothing more than a damp, uncleaned anode... it exhibits a typical
white smut that cleans off easily.
And the set itself:
Standard Titanium cathode. The efficiency had to be high. I didn't even chill the liquor prior to harvesting. HCl additions were occasional
wash-bottle squirts. If you want cheap MMO, that is as inexpensive as I've ever encountered.
Please, no one suggest that it makes perc. I'm not even going to try. If someone wants to know that badly, buy some yourself.
The ONLY drawback I found so far is the odd shape, and the fact that welding it is a bit more challenging, but no big deal. I have come to the
conclusion, though, that as cheap as this stuff is, I'd simply cut it in such a way that you don't NEED to weld a shank onto it. Simply cut it into a
useful shape and attach your DC current directly to the mesh.
Dann2, how goes the graphite cell? 
[Edited on 11-2-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
MMO MMO MMO MMO MOO!
Hello Swede,
Definitely MMO, no doubt about it.
MMO has come from a difficult to get thing, to being sold on ebay at a knockdown price and under the name of Ti mesh!
The Graphite will have to be renamed very-poor-mans MMO or perhaps Paupers MMO.
The Paupers MMO (PMMO) is going OK. There is little or no erosion visible on the anode though the liquid is getting black. There is actually very
little black suspension. Still taking samples and keeping pH at about 7.0. Temp. of cell is approx. 18C. Takes quite alot of acid when it is all added
up.
Some phots's below. The jam jar views consist of exibit one with a light behind the jar. The other one (exhibit two), no light behind the jar.
You can see the anode clearly in the other picture. There is very little erosion. There is no noticable thinning or erosion at the water line (not
shown in picture).
Dann2
LONG LIVE THE PMMO!!!!!!!

|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
nice results, I hooked up my 12 Amp cell yesterday, and within 20 hours I have a half an in of crystals built up at the bottom
i should have put the electrodes deeper so it would convect more, the top is so hot and the bottom still cool... ohhh well, its working.
and by the way, how much KCl can you guys dissolve. I tried for 330g per L, but it just precipitated out to about 280g per L i was using distilled
water.
how did you figure out your percent purity swede?
[Edited on 12-2-2009 by y2kbugger]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by y2kbugger
nice results, I hooked up my 12 Amp cell yesterday, and within 20 hours I have a half an in of crystals built up at the bottom
i should have put the electrodes deeper so it would convect more, the top is so hot and the bottom still cool... ohhh well, its working.
and by the way, how much KCl can you guys dissolve. I tried for 330g per L, but it just precipitated out to about 280g per L i was using distilled
water.
how did you figure out you percent purity swede? |
If you refer to the 99.3% I think I quoted in my blog, that number is suspect, and excludes contaminants other than chloride. What I did was took 10
grams of cell chlorate after it was washed and harvested, but BEFORE any recrystallization or other sort of processing, dissolved that in distilled
water, and used Hach chloride titration strips to determine how much chloride ended up in and on the raw crystals. A bit of math yielded the 99.3%
value. You could do the same thing using silver nitrate titration, or some other technique. Some of the other possible contaminants might be
chlorites, hypochlorites, etc, but given the total lack of any hypo/chlorine smell of both solid and solution, I'm fairly comfortable that the major
pollutant was chloride.
The process with MMO is so clean, I'm fairly confident that I do not need to recrystallize before feeding the chlorate into a perchlorate cell. But
if you wanted to, there's no doubt that recrystallization of harvested chlorate would result in a very clean product, probably close to 99.8%
The other conclusion I came to was that it is WORTH a thorough wash during harvesting. You'll lose product (but it gets recycled) and you end up with
cleaner chlorate.
I have no idea of the concentration of KCl for this particular experiment. I have a 5 gallon bucket that I filled 1/3 or more with KCl nuggets from a
big bag; it was then topped off with water, and allowed to sit for several weeks. When I need KCl stock, I simply decant. The concentration will
vary with the temp of the bucket. If I wanted to really max out a run, I'd saturate at the planned temp, say 60 degrees, and then lay the current on
fast and heavy to keep the heat up and prevent any KCl from crystallizing. After a day or so, if the heat has been maintained, enough chloride will
have been used to make the possibility of KCl crystal formation due to cooling off a bit a very small one.
I'm going to set up some sort of data-logger system and track a few runs. There's GOT to be some valuable data in the voltage, current, pH, and temp,
enough so that it should be possible to execute additional runs without constant fuss and monitoring of chloride levels, end of run conditions, etc.
Something along the lines of "when the voltage required for 50 amps is at 1.3X starting voltage, then the chloride should be at 8% by weight; time to
harvest..." Using data in this manner does require a CC/CV supply for meaningful results.
@Dann2 - it's amazing that the edges of your graphite anode are not more corroded. I too got the simple "feeling" that significant amounts of HCl are
required to maintain optimum conditions. I also get the feeling that proper pH is something we (as a group) have ignored for too long. My last big
run, with the T-Cell, was pH controlled, and the efficiency went way up, AND the environment seemed to be "sweeter" for lack of a better term... less
chlorine and hypo smell.
[Edited on 12-2-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
Came accross the following paper from:
http://www.chlorimax.com/pdfs/Effects_of_Electrolyte_Impurit...
It's describes (cut and paste):
Presentation provides an introductory review of the role
of electrolyte impurities in chlorate cells on DSA
coating and chlorate cell performance. Provides a
series of case histories discussing specific impurities
that have initiaited premature DSA failures in chlorate
systems. Also touches on the impact of impurities on
solution processes and cathode effetcs often mistaken
for changes in anode performance. Presentation is
speciifc to the chlorate cell industry.
It's a good read IMO AFAICS. Have not read it yet though.
It is attached below also.
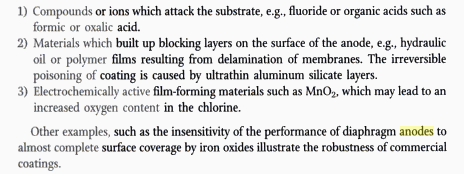
It says that Flourine is bad news for DSA. It attacks Ti.
The F may be bad news for LD Ti substrate anode too?
We sometimes use F in Perchlorate cells to stop reduction at the Cathode and raise the Oxygen overvoltage at the anode (ie. cell will make less
Oxygen).
You do have to laugh at the long life times of DSA though.
See their Technical section here:
http://www.chlorimax.com
for lots of info. on Nafion membrane and more if you should be so inclined.........
Dann2
[Edited on 12-2-2009 by dann2]
Attachment: Effects of Impurities in Chlorate cells on DSA.pdf (712kB)
This file has been downloaded 1020 times
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
yea that was the figure i was referring too. ha thats such a simple method of doing it i was looking at a chlorate titration:
Iodometrically
1. Treat the sample to remove hypochlorite, and dilute it such as to obtain a solution containing approximately 0.02M of chlorate.
2. Place 25 ml of the chlorate solution in a glass-stoppered conical flask and add 3 ml of concentrated hydrochloric acid followed by two portions of
about 0.3g each of pure sodium hydrogencarbonate to remove air.
3. Add immediately about 1.0 g of iodate-free potassium iodide and 22 ml of concentrated hydrochloric acid.
4. Stopper the flask, shake the contents, and allow it to stand for 5-10 minutes. Iodine is liberated according to the following reaction:
ClO3- + 6I- + 6H+ Cl- + 3I2 + 3H2O
4. Titrate the solution with standard 0.1M thiosulphate in the usual manner.
Using ferrous sulphate
1. Treat the sample to remove hypochlorite, and dilute it such as to obtain a solution containing approximately 0.02M of chlorate.
2. Place 25.0 ml of the sample solution in a 250 ml conical flask.
3. Add 25.0 ml of 0.2M ammonium iron(II)sulphate solution (Mohr's salt) in 2M sulphuric acid.
4. Cautiously add 12 ml of concentrated sulphuric acid.
5. Heat the mixture to boiling, and cool to room temperature by placing the flask in running tap water.
6. Now, either titrate the excess of Fe2+ with potassium permanganate or with 0.02M potassium dichromate with an indicator of 20 ml 1:1
water/phosporic(V) acid and 0.5 ml sodium diphenyl-amine-sulphonate.
seems a little complex.
well my anodes are doing fine, the MMO is holding up just as swedes did.
but.. my air vent hose connector came loose from the heat/corrsive fluid, and let my perfectly non corroded wire attachment get all oxidized.....damn,
my next one will be secured better
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello y2kbugger,
There is a Chlorate titration here. You probably seen it before. It's not too difficult to do. Works for me anyways.
http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/...
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I realize my method was crude and presumptive, but wouldn't the easier method be detection (mass determination) of everything that is NOT chlorate, as
opposed to determining chlorate concentration? The latter is important for obvious reasons, but I think by making simple presumptions, we can
simplify.
Destroy chlorites and hypochlorites, leaving chloride and chlorate ion. Titrate for chloride. Everything else is chlorate.
With modern MMO, the challenge isn't so much the chemistry, it is the physical construction of the cell, and the materials used, that presents the
greatest challenge, IMO. Cells tend to fall apart, chlorine is dumped, connections corrode at insane rates...
@Dann2, thanks for the resource.
Today I need to put some work into a thermocouple amplifier for the data logger. The only reason I'm going type K TC is because I have a lot of
hardware for it left over from a previous project, and the TC is useful for high-temp processes.
[Edited on 13-2-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
I would agree that there is nothing to beat the Chloride strips both for Chloride and (indirectly) for Chlorate.
When one goes over to MMO (it's not too long ago (or am I getting old) that bare pieces of Ti were hard to come by, MMO was more or less not
available) ) the 'bottle neck' of problems shifts to other things in the Chlorate business. The 'Anode problem' is no longer a problem, in fact, it
will probably be the last thing to give trouble in the cell (as pointed out by Swede).
I think there is very little Chlorites and Hypochlorites in a Chlorate solution. A one or two percent. Not too sure. Does anyone here know how much
exactly?
Giving the solutions a good boil gets rid of most of them AFAIK.
Dann2
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
What I am thinking at this point is that if a PVA thickened
hydrosol of peptized hydrated Bi2O3 and SnO2 is applied as a dip and bake coating to the MMO , maybe three or four coats, what will be the result is a
perchlorate anode.
See example 1 and 2 of the attached patent US6777477 and modify example 2 following the increase of PVA to 0.87 g as
described for example 7. The level of Bi doping could also probably be raised, perhaps doubled. A very small amount of included Antimony doping on the
order of 1/2% to 1% would probably result in a finer grain structure for the baked coating. That should get the job done. This treatment could perhaps
be finished by a dip in ammonium fluoride and baking, although this is my speculation concerning the fluoride treatment which should further toughen
and dope the coating.
Really this should be done for an oxygen barrier before electrodeposition of PbO2, to change the overvoltage gradient for the coating layers in a way
that opposes
delamination of PbO2 by any permeation of electrolyte
which could evolve oxygen at a lower voltage than the
outer surface PbO2, spreading across the interface of
the PbO2 and MMO otherwise to separate the layers.
As the MMO is catalytic it would tend to preferentially evolve oxygen from any permeating electrolyte, unless it has been coated with something like
Bismuth or Niobium doped SnO2
in order to raise its oxygen overvoltage higher than the
the overlying PbO2.
Hmmmm, it's just about time for a Valentine's Day
diva break  So my fellow estrogen admirers ..... So my fellow estrogen admirers .....
http://www.youtube.com/watch?v=7fpkF31MQ30&fmt=18
http://www.youtube.com/watch?v=XVQcvCfi4G8&fmt=18
[Edited on 13-2-2009 by Rosco Bodine]
Attachment: US6777477 Bi2O3 doped SnO2 via ammonia soluble derivative.pdf (67kB)
This file has been downloaded 662 times
|
|
|
jimwig
Hazard to Others
  
Posts: 215
Registered: 17-5-2003
Location: the sunny south
Member Is Offline
Mood: No Mood
|
|
mmo's available via ebay = $39 american
mmo's available via ebay = $39 american
after all the hassle to do with DIY this seems a very reasonable solution.
but i could be wrong.
craZy jiM wGGns
--packrat, professional bum. -- once just tired
now REtired.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Graphite Erosion
Hello Folks,
Ye old PMMO (Graphite) cell is starting to look like a classic Graphite Anode cell. Jet black electrolyte that you cannot see through even when there
is a light behind the cell or container.
Some photo's below. I did not bother with the photo of some of the cell contents in a jamjar with a thermometer (as before) as you cannot see the
thermometer at all.
The four samples in the picture below do not tell the whole story as they are being taken from the cell without vigourisly stirring the cell. If I
stirred the cell, the last three samples woud be much darker, especially the last one. The first sample would be similar to what you see as there was
no black sediment on the bottom of the cell at that time.
Samples are from 9th, 12th, 13th and 15th (February).
The anode has eroded more in the last two days (14th and 15th) than it has in the previous 20. It eroded more in the previous two days prior to 14th
and 15th (ie. 13th and 12th) that it eroded in the previous 18.
I think Perchlorate is about to start forming as I can see a greenish efflorescence that you get when there are tiny amounts of Perchlorate when using
Methylene blue to detect it. Could be wrong though.
The cell has entered a different 'mode' as I have the acid turned off for the last four days and the pH is staying at 6.7 approx. Temperature is still
in the region of 20C.
Dann2
[Edited on 16-2-2009 by dann2]

|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Interesting if somewhat unfortunate results, Dann2. What I find intriguing is the fact that the system stabilized at pH 6.7 with no further acid
additions needed. As you say, it entered a new phase of chemistry whereby the graphite began to erode at a rapid rate, and the pH did not climb.
What do you think the chlorate concentration would have been if you'd pulled the plug before the graphite began to erode?
I think the answer to the graphite cell conundrum is to either be satisfied with stopping the process at a higher chloride concentration, or, if you
don't mind replacing anodes, investing in or working on an effective and large vacuum filtration system that would truly clean the dirty liquor.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
I have taken a sample from the cell every two days or so. All will be reveiled when I titrate them. The Anode erosion was expected when Chloride got
low. I don't expect large CE since the cell was so cold. Next run will be at approx. 40C, I will insulate the cell and hopefully it will heat up to
about this Temp.
No Perchlorate yet. I will pull the plug on the cell as soon as it starts forming......if the anode lasts that long.
I think that it will be easy to get rid of the black stuff. Boil the liquid to get rid of dissolved gases and then let the whole lot settle for a few
days and decant off the (hopefully clear) liquid. I found before that if you do not boil the liquid that the gases are inclined to keep the solution
agitated as they appear out of the solution over a period of days.
Dann2
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I suspect the erosion of the graphite may be self-catalysing and that the more of the erosion product is in suspension, the greater is the erosion
rate encountered.
Has any erosion rate comparison been done for a pumped electrolyte where the eroded graphite is filtered out of the electrolyte continuously?
I also suspect that when the pH stabilized may mark the
effective practical endpoint as Swede alluded to above.
[Edited on 16-2-2009 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
Aqua_Fortis 100% sent me a patent showing how Graphite could be treated with Iron (IRON, FFS  ) . ) .
I pasted it here as there is some info. regarding cell voltages in relation to Chloride concentration. I though this might interest yourself Swede if
you were trying to link cell voltage with Chloride concentration. The cell voltage does not change very much according to the patent and it would be
very temperature dependent. Connections would have to be very very good too.
________________________________
Abstract: (US3632444)
For example, during a comparative experiment employing a normal chlorate graphite electrode with an iron impregnated chlorate graphite electrode, as
the concentration of NaCl decreased from about 250 grams per liter to about 150 grams per liter, the cell voltage increased from about 3.25 volts to
3.6 volts with the normal graphite while with iron impregnated graphite, the increase in cell voltage was from about 3.15 to 3.35 volts
__________________________________
Anyhow my Na Chlorate cell registered Perchlorate with a Methylene blue test and I turned it off. That was a few days ago. Still have to titrate
contents of samples. I think the Chloride concentration was quite low before the Perchlorate appeared. The anode was eroding at an unacceptable rate.
It eroded more in the last day of operation that in the previous 22 days of operation!
I cranked up a KCl cell using the same anode. I scraped and scrubbed all the loose Graphite off the Anode so it was like new Graphite. Some pictures
below. You can see the clean Chlorate but that is only on day three. It will get slightly blacker as the days go by, but not much. I do not intend to
run the cell to the Perchlorate point.
The lesson with a Graphite anode is: Controll the pH. In our case this really means adding acid at a certain rate and keeping an eye on pH.
The Chlorate is gathering on the Anode alot. Perhaps I am geting too much 'Anodic Chlorate formation' at the low temperatrue (20C) that the cell is
running at.
When starting the cell I measure the pH of the Chloride solution, it was 6.4.
I added 3cc 12% HCl before current was started to give the acid a bit of a head start. This seems necessary from my experince with the Na cell. I also
turned acid on at the normal rate. After some hours there was a stink coming from the cell and on measuring the pH it was 0.30.!! Turned off acid for
5 hours and pH was then mearured at 7.6. I decided to add acid to take pH to 6.8 and I had to add 20ml acid (quite a lot) to get pH to 6.8. There
seems to be some buffering going on. A very unruley cell!!!!!!!!
The pH stayed at normal value with normal acid addition from then onwards.
It would appear that it is not a good idea to add acid at the start. Just start pump and leave alone.
Dann2

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Some results of Na, pH controlled Graphite anode cell below.
<table border><tr><th colspan=2> Graphite Anode with pH control @35mA/cm<sup>2</sup> @ 20C</tr>
<tr><td>From Days</td><td>% CE</td></tr>
<tr><td>0 to 1</td><td>16</tr>
<tr><td>1 to 2</td><td>36</tr>
<tr><td>2 to 4</td><td>30</tr>
<tr><td>4 to 6</td><td>38</tr>
<tr><td>6 to 9</td><td>41</tr>
<tr><td>9 to 11</td><td>37</tr>
<tr><td>11 to 14</td><td>36</tr>
<tr><td>14 to 18</td><td>33</tr>
<tr><td>18 to 20</td><td>19</tr>
<tr><td>20 to 23</td><td>19</tr>
</table>
The cell was approx. 2.2 liters with 5 amps (varied a bit) going into cell.
The solution was clear up to day 7, (approx. 150 g/l Chlorate).
Very very little actual Graphite in the solution and not at all visable when a light was put behind the cell.
At day 17 (approx. 500 g/l Chlorate) the solution was starting to get black but still very little actual Graphite in the solution. No black visable
when light put behind a jar of solution.
There was little or no visable erosion on the anode at day 19 (520 g/l Chlorate) but the solution was gettng very black at this stage with a light
unable to shine through it.
At day 20/21 (550 g/l Chlorate) Anode erosion started to become severe with the deterioration of the anode visable.
At day the end of day 23 Perchlorate appeared. At this point the Chloride concentration was 25 grams per liter calculated from the fact that the
starting solution was 334 g/l Chloride and the Chlorate concentration was now 581g/l.
The CE is pathetic.
Anode erosion is good.
More heat needed I guess/hope.
Currently running a KCl cell as shown above in a pic. I will just weigh the product in a few days and get overall CE. There
will be no titrating.
Next NaCl will be insulated to take temp. up to 40C or so.
Acid into cell was 1.8ml per hour for first two days.
Then decreasing to 0.6 over the next 4 days and left in that region untill stopped.
Sometimes I addes some acid manually if I though pH was getting away. Sometimes I turned acid off for some hours
if pH seemed to drift low. Not very scientific!
Acid was stopped on day 18.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Ran the pH controlled K Chlorate cell for 7 days and pulled the plug.
The K Chlorate sticks to the Anode somewhat but it does not seem to be a big problem. Acid addtions seem to be less that an Na cell as I was able to
turn off the acid for the last day and a half. Very low acid addition on day 5/6.
Cell was more or less the same as the Na cell above. 20C, 4.5 amps into cell (similar CD).
Extracted the Chlorate by cooling solution and running through a cloth. Most of the black (there was some) stayed with the water leaving the Chlorate
fairly clean. You could probably use the Chlorate as it is, though it is off white. In practice you would probably recrystallize to get rid of any
Chloride and clean the Chlorate up in the process.
Test for Perchlorate, there was none.
The pH controlled Graphite Anode cell is not in the same league of cleanlyness as an MMO cell but it is not far off! There seems to be more black
stuff compared to the Na Cell (at day 7) but that may be because the Anode was used before. There is a picture above of Chlorate sticking to anode.
When the Anode was placed back into the cell all this fell off and seemed to take a layer of Graphite with it. There is not much erosioin product
though (few grams at most) and whatever was there settled to the bottom of the mother liquor and left a 100% clear solution (for recycling after
decanting).
Total weight of product extracted was 422 grams. 842 amper hours into cell gives a current efficiency of (drumm roll) 66%. At least it's better that
the Na cell.
I cranked up another Na cell. I insulated (wrapped in swaddling cloths) the cell using tinfoil and fiberglass + polystyrene board underneath. The
temperature went to 50C (a bit too high, but I am going to run it at that ). Current is higher because cell is at a lower resistance because of the
heat I guess.
Dann2
[Edited on 26-2-2009 by dann2]

|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Some excellent postings, Dann2. I'm surprised the efficiency of the potassium cell was better than the one starting with sodium salts. Have you
tried recrystallizing a portion of that chlorate? I wonder how difficult it would be to get the graphite coloration out of the crystals. You'd
probably need to hot-filter the near boiling liquor through a fairly tight filter (assisted by vacuum) to get rid of those microscopic particles, and
as you mentioned, it's probably not necessary for compositions using chlorate.
I'm hoping that collected data can in fact help determine the cell constituents despite what that patent says. When they say "fairly constant"
voltage, I wonder what their definition of that is? With a constant current supply, I've seen the voltage consistently follow a predictable path,
but you are right, it IS temperature dependent, and it is also quite dependent upon the level of the liquor, especially if your electrode surface
areas decrease as the level drops. For the sake of consistency, some sort of top-off arrangement is a must; OR, engineer the system in such a way
that the electrodes are deep enough so that liquor level changes have a minimal overall impact.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
I will see if Chlorate will clean up. I think it will clean up OK by filtering.
The black stuff that is in a Graphite Anode cell that has not been let run and run seems to be more easy to get out of the liquor that in a cell where
a large amount of(Graphite) Anode erosion has been allowed to take place.
The Na cell (large amount of Anode erosion) liquor is still sitting in a glass container but the liquor has still got a deep black/oragne colour to
it. There is also some very very fine Graphite on the bottom of the container which stirres up very very easily. I will be very difficult to clean
it up unlike the Graphite (small amount) that is in the K cell where the small amount of Graphite seems to have a larger particle size and falls to
the bottom of the liquid. This liquid can be decanted off or (IMO) will filter fairly easily. There is some Graphite trapped in the solid K Chlorate
but (IMO) it has a fairly large particle size and will filter out unlike the black discoleration in a cell that has had far far too much Graphite
Anode erosion (Na cell in my case).
In a cell that has had large amounts of Graphite erosion, it is as if the Graphite is 'dissolved' in the liquid. Perhaps some compound forms?
[A rather long winded explanation of what I am trying to say...........]
About the voltage accross the cells, it may also be Anode age dependent. As a (say Graphite) anode ages it gets pitted and has in effect more surface
area giving different Voltages. Same may even apply to Cathode. Sometimes deposits build up on the electrodes which may not help either. May not make
much difference though.
About the liquid level dropping and changing the Anode and Cathode surface area. If you are using a Ti runner down to an MMO anode then the Anode will
be effectively below water level all the time so long as you have the runner long enough (MMO one inch (say) below surface). The runner will not
conduct to the solution as it will be Anodized with TiO2. The problem occurs with the Cathode. You could cover the runners/Cathodes down to the
Cathodes with plastic tubing so that they are covered a half inch (say) above and below the nominal level of the cell. The cell volume can then change
up or down a half inch without the electrodes 'seeing' the change in level. The same applies to Anode that have not got runners going down to them.
Since you will be using MMO Anodes I guess this does not apply.
I also wonder if you could or should put a heater and controller into cell to keep temperature steady. Perhaps keeping it cool may be more of a
problem.
If the cell was force heated to (say) 70C with a controller it can be held steady at that point. If you have constant temperature for a few runs and
collect data then you could perhaps later factor in the changing temperature stuff.
The data collection stuff on a cell was always something I wanted to do (and others) but was too lazy and miserable (not willing to spend $$) to get
around to it. The stuff you purchased (apcForums) is definitely the biz.
Measuring density of solution might give useful data. Not too easy to do with a computer I guess. Dissolved gases may make it useless data though.
I wonder will you see a small Voltage variation each time your pump puts in a quantity of acid..........
Keep up the good work!
Cheers,
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | | About the liquid level dropping and changing the Anode and Cathode surface area. If you are using a Ti runner down to an MMO anode then the Anode will
be effectively below water level all the time so long as you have the runner long enough (MMO one inch (say) below surface). The runner will not
conduct to the solution as it will be Anodized with TiO2. The problem occurs with the Cathode. You could cover the runners/Cathodes down to the
Cathodes with plastic tubing so that they are covered a half inch (say) above and below the nominal level of the cell. The cell volume can then change
up or down a half inch without the electrodes 'seeing' the change in level. The same applies to Anode that have not got runners going down to them.
Since you will be using MMO Anodes I guess this does not apply. |
Good info and a nice idea. There are a few sources for PTFE heat shrink tubing I've been wanting to tap for some time now, and that would be a good
use for them.
I've often thought too about measuring specific gravity of the liquor and seeing if it varies enough during the run to provide useful data. Again, a
temperature-dependent measurement, and you'd probably need some VERY sensitive hydrometers or some other method, such as a volumetric flask and a good
scale.
I suspect on a streaming chart of cell voltage that you would in fact see a nice "blip" in the downward direction as the acid is injected. Then, the
voltage would creep back up to "normal" as the acid is spent in neutralizing whatever species it does.
I'm hard at work on transducers and signal conditioners. It's been a bit trickier than I thought it would, but nothing insurmountable. When the
smoke clears, I should have transducers for cell voltage, current, temperature (PT100 RTD for good accuracy, or type K thermocouple), and possibly pH.
THAT will be the tough one, requiring clean amplification and filtering to prevent noise from making a hash of it.
We've discussed pH electrodes before in the context of continuous immersion in a chlorate cell (my hypothesis: it will be fatally poisoned in a matter
of hours) but it'd be worth a cheap pH electrode or two to see if it does in fact die a quick death.
That lead dioxide anode I made, the good one, is still sitting in its ziploc bag, awaiting testing! I need to finish up the data thing first, then
more LD and other anode fun. Question: Do you guys think my LD plating solution will store well? Everything I've read says "no." 
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
Would an ISFET electrode stand up any better than a glass/AgCl one? Or would it be another more expensive sacrifice to the gods?
I'd be leery of putting an electrode into an active electrolytic cell - unless the pH meter was totally isolated from the electrolysis supply, I'd bet
on some current flowing through the pH system and probably giving erroneous readings. At worst, it could destroy the electrode and possibly the meter.
If you had a pump or other means of taking more-or-less continuous samples which were not electrically connected to the main bath it might be safer. I
would be sure that there was not a continuous path through liquid by (for example) dripping the sample into a beaker and siphoning it out in drips to
be sure that the flow was broken.
|
|
|
| Pages:
1
..
20
21
22
23
24
..
48 |