| Pages:
1
..
20
21
22
23
24
..
40 |
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Wow, SO3 is an amazing substance, I always wanted to make some and do some nitronium sulphate-based nitrations  I don't have the equipment to do so, though. I don't have the equipment to do so, though.
Rest In Pieces!
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
It seems there isn't an appropriate thread for showing all kinds of weird stuff found online, but this is the closest one here because there are
photos inside this link.
Adrian's Home Chemistry Lab
If this is one of you, guys, I'm sorry for my ignorance. It's just that I'm amazed by the awesomeness of this lab. Not only it looks like a 19th
century lab where some guy discovered a chemical element, but it even has a freakin' organ inside to add some general spookiness. 
The videos are really cool, do check it out.
One thing scares me a bit - I don't see a ventilation system and it seems like the lab is a neat place to slowly poison yourself as the years go by.

Also, I wouldn't keep a neat and expensive thing like an organ inside a lab where corrosive fumes linger around...
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
nitronaphtalene (I think)
full size (4000x3000)
http://img165.imagevenue.com/img.php?image=467150715_IMG_258...

|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Really? I thought it was a lemon peel... now I have a better look I see. 
[Edited on 24-9-2012 by Eddygp]
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
Hehe. I dissolved the nitronaphtalene in a bit of acetone and let it evaporate. The interesting thing is that only the bottom of the petri dish was
covered with the liquid - there was just a few mililiters of it. And yet the solid somehow climbed the container's walls.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by learningChem  | | Hehe. I dissolved the nitronaphtalene in a bit of acetone and let it evaporate. The interesting thing is that only the bottom of the petri dish was
covered with the liquid - there was just a few mililiters of it. And yet the solid somehow climbed the container's walls. |
That's called an evaporative creep. Usual nuisance.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

Two small bromine apoulles and a small flask with the rest of the bromine what I have used in a reaction.
Each ampoulle contains 25-30g of pure Br2, wouldn't be funny to break one 
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Yummy Bromine. Keep up good work with the pictures
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Ego_and_his_own
Harmless

Posts: 17
Registered: 27-9-2012
Member Is Offline
Mood: Curious
|
|
Oh this will be my topic. I love photography and i came to love with chemistry. 
|
|
|
Ego_and_his_own
Harmless

Posts: 17
Registered: 27-9-2012
Member Is Offline
Mood: Curious
|
|
(warning: site flagged as malware vector by Google)
http://darkobulatovic.com/photos/P1010248.jpg
Here is Sulfamic Acid after overheating and sudden change to this solid (amber like) state after cooling down in vacuum.
Bubbles of gas got trapped inside of it.
Weird.
[Edited on 7-6-2013 by Polverone]
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
It turns out that making NO2 is easy. I used a TV circuit high voltage line, a couple of canadian 25 cents (pure nickel). I also added a small cup of
water to absorb the NO2 and make HNO3.

|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
>That's called an evaporative creep. Usual nuisance.
Oh. Thanks for the info.
|
|
|
CMOS
Harmless

Posts: 14
Registered: 27-2-2009
Member Is Offline
Mood: No Mood
|
|
acetamide


|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
A polished piece of Magnalium

|
|
|
rstar
Hazard to Others
  
Posts: 138
Registered: 22-9-2011
Location: Besides valence shell
Member Is Offline
Mood: Dark
|
|
nothing much to show...
just copper pieces, with my low quality camera 

"A tidy laboratory means a lazy chemist "
- Jöns Jacob Berzelius
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
my first lead acetate crystals! lead was dug from a hillside where we go shooting.

|
|
|
sainandrew92
Harmless

Posts: 7
Registered: 15-10-2012
Member Is Offline
Mood: No Mood
|
|
Evening all.
I'm new to the forums and figured I'd just introduce myself with a picture.

Italian might be familiar with this stuff based on his previous posts.
Iron Acac crystals. Also, I have Copper Acac but it's kinda boring to look at.
man will occasionally stumble over the truth, but usually manages to pick himself up, walk over or around it, and carry on.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
More nice pictures!
Here is a pumpkin elephant toothpaste picture I found

[Edited on 19-10-2012 by mr.crow]
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by vmelkon  | It turns out that making NO2 is easy. I used a TV circuit high voltage line, a couple of canadian 25 cents (pure nickel). I also added a small cup of
water to absorb the NO2 and make HNO3.
|
how to make this, what should I take out from tv?
by the way, which safety precautions you take with this?
thanks
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Search for TV Flyback driver. It is the black transformer the red high voltage wire from the CRT comes from.
Its an easy project to re-write the primary to get some really high voltage. High voltage can easily break down insulators and kill you. A neon sign
transformer would work too and is current limited.
The heat from the arc breaks down nitrogen and oxygen into NOx very inefficiently.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
There are some ‘arc chemistry’ examples on this forum if I recall well and they don’t use high voltage sources, rather they use grid power (110
or 220 V AC) with a suitable reactance to limit current. See this old classic for an electric furnace:
http://www.sciencemadness.org/talk/viewthread.php?tid=2492&a...
and:
http://walkermetalsmith.com/reprints/Portable-Arc-Furnace.pd... (example of reactance near the end of the article)
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
A certain charm
These aren't necessarily "pretty" but I wanted to post them anyways. I always thought chemical containers from back in the day have a certain charm to
them...


Compared to today's modern bottles...
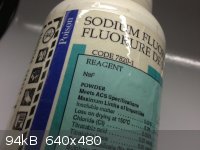
[Edited on 27-10-2012 by Mailinmypocket]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Some recently made white P, cleaned with chromic acid.

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
Happy Halloween.


Victor Grignard is a methylated spirit.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
that is so cool!
[Edited on 10-29-2012 by cyanureeves]
|
|
|
| Pages:
1
..
20
21
22
23
24
..
40 |