| Pages:
1
..
19
20
21
22
23
..
40 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
arclight, please post details in another thread. I'm interested in exactly how you produced the Cu mirror band.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|

link to large version
link to video
If anyone can determine the species, or at least genus, that'd be great.
|
|
|
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
Might want to try bugguide.net
You can post an ID request or browse their image gallery and try to find a match (could take forever, they have tons and tons of photos).
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Liparus glabrirostris?
Rest In Pieces!
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
I am currently crystallizing some caffeine citrate, it has so far made some absolutely beautiful hairlike crystals. Still a bit of solution to dry
though, will post pics for sure when its ready.
EDIT:
Here ya go.

[Edited on 27-8-2012 by liquidlightning]
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
It's uncanny how similar to hair they are.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Something interesting:
LIQUID OZONE!
Just made some with an ozonisator, condensed in a test tube with some liquid nitrogen. There was no problem with it until we started the reaction.

The nitrogen/acetone mixture solidified the deuterochloroform what was used as a solvent, the ozone condensed on it and it reacted explosively with
the alkene what would have been ozonized. And then: bang.
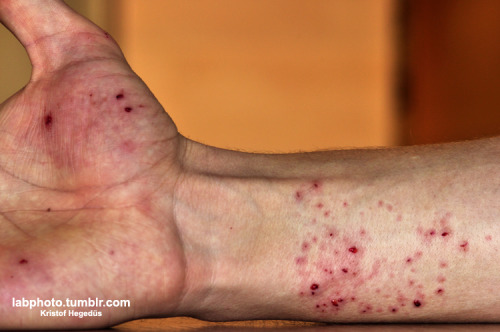
Never misjudge the power of chemistry. And never forget to use safety glasses!
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Wow, liquid ozone? That is amazing, I always wanted to see that! I am sorry for your injuries, though :/
Rest In Pieces!
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
Verg they were very hairy early in the crystallization, but they kinda got bunched while getting them out...
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Nice, I had read that it had a deep blue color but I had never seen ozone.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
kristofvagyok, hats off!!!
This is amazing, there are no photos of liquid ozone on the entire Web, and I haven't seen it in books, either.
I'm sorry about the mishap, I hope your hand is ok, but well done! This is the real thing. Just... wow!
If that's from an ozonizer, it's not pure ozone, am I right? After all, LN2 should've freezed it.
Or you've managed to remove excess oxygen and nitrogen from the air? Have you fed the machine with pure oxygen?
I'd seriously consider putting this photo on Wikipedia for everyone in the world to see.
[Edited on 28-8-2012 by Endimion17]
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
My friend Ivan @ Periodictable.ru made this before:
I'll ask him to post it on Wikipedia 

|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
What is this strange solution?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
they were just talking about ozone. that would be my guess.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Um, I don't think that blue liquid is ozone...
I'll take a guess: A copper-ammine salt. Or cobalt. Or... I give up, what is it?
EDIT: That is liquid ozone, isn' it? I feel stupid, I was kind of expecting a paler blue color from ozone, like liquid diatomic oxygen.
[Edited on 28-8-2012 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
actually, the only other picture (claimed to be) of liquid ozone i have ever seen was a miniscule amount and it was much darker than these samples.
kristof, did you manage to get all of the glass out of your hand? sorry for your pain, sir. i know that sucks, but you did share a wonderful image of
a chemical most people will never see in their lifetime. thank you for that!
[Edited on 28-8-2012 by Rogeryermaw]
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  | kristofvagyok, hats off!!!
This is amazing, there are no photos of liquid ozone on the entire Web, and I haven't seen it in books, either.
I'm sorry about the mishap, I hope your hand is ok, but well done! This is the real thing. Just... wow!
If that's from an ozonizator, it's not pure ozone, am I right? After all, LN2 should've freezed it.
Or you've managed to remove excess oxygen and nitrogen from the air? Have you fed the machine with pure oxygen?
[Edited on 27-8-2012 by Endimion17] |
Well, at first, I want to tell, theat this was not my hand, it was one of my friends who had made this reaction instead of me, because I had another
one at this time... But he is okay now.
This explosion was a pity, the glass just "evaporized" to a lot other small pieces (it was 5ml heart shaped flask) and some of it landed in the hand
of my friend... It is not serious, it will get better in 1-2-3 days.
The ozone what was produced wasn't pure, it was made from 99,994% oxygen and this contained just a few percent pure ozone.... So the color of the
solution is thiswhy so "bright blue".
The accident could be caused by a lot reaction, but the most realistic is that the liquified ozone/oxygen mixture just went off..
A bit offtopic, but does anyone know that how that ozone was produced first time ever in laboratory? It is an interesting reaction, what I have never
thought about. I will post it soon(:
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Well, the color does seem to be too bright, and it's from an ozonizer, therefore I concluded it's not pure ozone. The standard description is "dark
blue liquid", and pure ozone can be solidified using LN2 (I suspect solutions of O3 in O2 have a lower melting point) into a dark blue, purple black
solid.
I don't think the quantities made for official experimenting were a lot larger than what it fits into a tiny test tube or perhaps absorption cell for
spectroscopy. It's simply too dangerous.
I'm acquainted with periodictable.ru, the guy makes great photos and videos, but I've never seen his photo of liquid ozone before. I'm subscribed to
his channel on YT, but it's been a long time since he made any videos...
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
It's O4 
@Endimion- I work with him closely, I share some of my projects with him and vice versa, and I relay orders on his behalf.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Liquid Ozone? Wow!! If any of you have played Eve Online you will know liquid ozone powers cyno fields, but I didn't know it existed in real life
I have made liquid oxygen before and its a nice pale blue.
Endimion17: You have to choose the lesser of two weevils
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mr.crow  | | Liquid Ozone? Wow!! If any of you have played Eve Online you will know liquid ozone powers cyno fields, but I didn't know it existed in real life
|
A really useful reaction is based on ozone gas and highly concentrated ozone solutions, this reaction is called ozonolysis(:
And what I have asked and noone replied: the first method for the preparation of ozone in laboratory was made by reaction white phosphorus and dry air
(or pure oxygen) in a sealed container. The reaction is spontaneous and ozone concentrations could reach up to 25-30%. I think it's an interesting
fact, I would have never thought about this reaction could occur(:
And the picture of the day:

-a nitration's product crystallized at the bottom of the breaker.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
@kristofvagyok; What is the reaction mechanism for this method of producing ozone? Does the phosphorus burn or just glow?
hibernating...
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Absolutely love the images of liquid ozone, as nearly all of your other photographic work, Kristofvagyok.
I did find one other image of this stuff on the net, and it is much darker indeed:

-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
kristofvagyok, I remember reading about that ozone producing method in one very old book on inorganic chemistry. I don't remember the name of the
book, but I think it was printed in Great Britain. What's your source?
Photos - just... wow. 
|
|
|
plastics
Hazard to Others
  
Posts: 141
Registered: 6-11-2009
Member Is Offline
Mood: No Mood
|
|
My tube furnace releasing the last molecules of SO3 from NaHSO4 at 820 Celsius

free picture hosting
The final result

keep photos online
|
|
|
| Pages:
1
..
19
20
21
22
23
..
40 |