| Pages:
1
..
19
20
21
22
23
..
30 |
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
methyl benzoate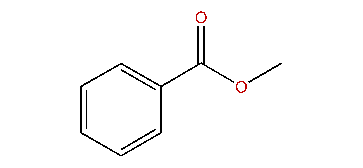
benzyl formate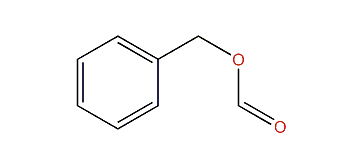
phenyl acetate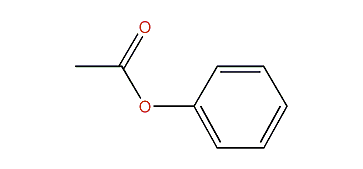
phenyl acetate would be a closer match for methyl benzoate, because it has both a phenyl and a methyl extremity, with an ester in between. inverting
the direction of the carboxylic group can result in similar physiological activity: compare the opioids pethidine and MPPP:
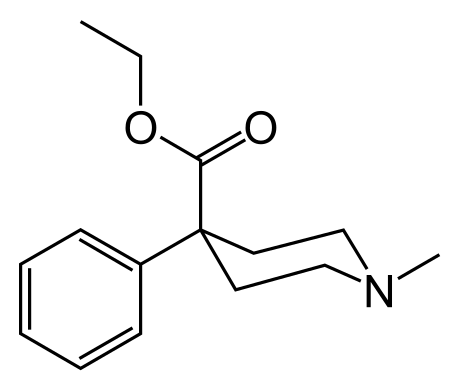 
OTOH a carbonyl extremity would probably impart a different behavior.
[Edited on 15-10-2012 by tetrahedron]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
This seems a little more involved than a short question
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I've just seen a redox equation involving manganese in varying oxidation states, and mid-way through the equation "Đ" is seen. In this instance,
what does it represent?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hexavalent  | | I've just seen a redox equation involving manganese in varying oxidation states, and mid-way through the equation "Đ" is seen. In this instance,
what does it represent? |
Example? It might be an abbreviation.
|
|
|
Boron Trioxide
Harmless

Posts: 42
Registered: 18-6-2012
Member Is Offline
Mood: No Mood
|
|
I have a few short slightly related question, answers to any or all would be appreciated.
-In the thread regarding MMO Electrodes it is said that MnO2 on its own is " oxygen selective anode coating" and will not produce chlorates, only
oxygen, is this true?
-Is chrome attacked by nitric acid?
-When producing nitrates, of specifically manganese or cobalt, will lower concentrations of nitric acid work or does it need to be 70%?
And less importantly
-Are there any other solvent aside from MEK that will dissolve PVC?
Thanks for any answers 
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
I scanned this but I don't know how much this will help you- I find it to be very useful... There is obviously a difference in attack vs. dissolution
but it might be helpful.
Attachment: Chemical Resistance Chart.pdf (1.8MB)
This file has been downloaded 955 times
As for the chromium? No reaction with nitric acid. It passivates. Just for fun I did a quick little demo here 
70% HNO3, along with a dumped out container of pure Cr.

Threw a bit of Cr in the HNO3.... No reaction.

And of course, to avoid wasting the HNO3, who could resist....Copper turnings!!!!(you can see the nitrogen dioxides running over my skin, so nice!)

[Edited on 3-11-2012 by Mailinmypocket]
[Edited on 3-11-2012 by Mailinmypocket]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Can someone tell me how to use url shortcuts of the type: /i5jthom /4gb1zjt ?
@kmno4 ?
Thanks
CRX
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Conrad
Harmless

Posts: 9
Registered: 14-11-2012
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Hi!
I recently bought a small bottle of phenylacetaldehyde.
Now, after 2 weeks in the chemicals cabinet, the bottle has grown half full of crystals.
I know my benzaldehyde loves to do this and turns into benzoic acid quite fast when not stored properly.
So, now comes the question:
Do I have a bottle half full of phenylacetic acid?
Does PAA build up so quick?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Read the instructions at http://tinyurl.com/. They're not the only service in this category, FYI.
I recommend against using these if your links have any kind of archival value. By concealing the actual URL, you prevent people from using archive.org
or other time machines to find the content if either the site itself or the redirector dies.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Would it to be possible to have an aqueous solution of just one cation or anion - e.g. Na+ or Cl- with no other cations or
anions present?
I have no use for it nor can I imagine one, this is simply out of curiosity.
[Edited on 16-11-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hexavalent  | | Would it to be possible to have an aqueous solution of just one cation or anion - e.g. Na+ or Cl- with no other cations or
anions present? |
Charge balance. Electrostatic force.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
http://courses.chem.psu.edu/chem36/Experiments/Exp84.pdf
Can EtOAc be substituted for ether during the washing??
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
Yes, but it's suboptimal. EtOAc is more polar, more soluble in water, and has a higher boiling point. I do not know the solubility of methyl benzoate
in EtOAc, but I do know that it is miscible with diethyl ether.
EDIT: And, from this site, water is more soluble in ethyl acetate than in ether.
[Edited on 23-11-2012 by Vargouille]
|
|
|
maxpayne
Hazard to Self
 
Posts: 78
Registered: 15-11-2011
Member Is Offline
Mood: No Mood
|
|
Solubility of compunds and it's behaviour
I have a 2 questions, and would appreciate if someone answer:
1. If I have two compounds dissolved in water, and one of it is soluble in non-polar solvent, will this other compound 'almost' completely go to that
non-polar solvent if I add it? Please explain.
2. If I have a compound A dissolved in a solvent X, and then I add another compound B which is more soluble in given solvent X, will compound A get
out of the solvent X if I saturate it with compound B? (salting out??)
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
To answer the first question, it won't be that simple. If you have a substance that is soluble in water and in a non-polar solvent, and it is more
soluble in the non-polar solvent, you will have to preform multiple washes, with vigorous mixing, mind you, to extract the majority of the substance.
With each wash, a little more of the substance goes into the organic phase, and eventually, with enough washes, there will be very little of the
substance left in the aqueous phase.
To answer your second, only if compound A is less soluble in a solution of compound B, or through the common ion effect. For example, isopropanol can
be salted out by addition of sodium chloride, which raises the polarity of the solution so that isopropanol is less soluble in the solution. If it
works by the common ion effect, compound B is a salt that shares a cation or an anion with compound A. Then, the addition of whichever is shared will
force the equilibrium of the dissolution of compound A to the left. For example, assume that A is NaCl and B is HCl. The addition of HCl increases the
concentration of Cl-, shifting the following equilibrium to the left, according to Le Chatelier's Principle:
NaCl(s) <=> Na+(aq) + Cl-(aq)
Thus, NaCl will precipitate if it is concentrated enough. Read up on the solubility product (Ksp) for the math behind it.
[Edited on 24-11-2012 by Vargouille]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I recently ordered some copper sulfate from the internet, and when it arrived, I noticed that it had many small, off-white particles in it. I
recrystallized it successfully (including a hot filtration, as there was some insoluble junk at the bottom of the vessel), and decided to make some
tetraamine copper (II) sulfate with what remained in solution.
So, I added concentrated ammonia until no more copper hydroxide formed, and then continued adding until it all complexed back into solution. I then
added ethanol until no more precipitated out, and then filtered out the lovely blue crystals.
What have I made, though? Tetraamine copper (II) sulfate, or tetraamine copper (II) hydroxide? Or a mixture of the two, perhaps?
Any help would be greatly appreciated.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
Tetraaminecopper(II) sulfate. Tetraaminecopper(II) hydroxide (Schweizer's Reagent) decomposes in alcohol. I made Schweizer's Reagent once before, by
creating Cu(OH)2 and dissolving in NH3. If I recall correctly, this looked somewhat different from a mere tetraaminecopper(II) solution. In any case,
this is a quote from my thread on the topic:
| Quote: | | The supernatant is drawn up in a pipette, and added to an excess of Crown Denatured Alcohol (a mixture of 65-75% MeOH, 20-30% EtOH, <10% IPA, and
<10% methyl isobutyl ketone), to precipitate the Schweizer's reagent in the same way as an amount of tetraaminecopper (II) sulfate was isolated
previously. This resulted in a white precipitate with a slight blue tinge. More of the supernatant is added, and the precipitate begins to go yellow,
eventually becoming a tan color. An exotherm may have occured, but it is unclear due to high ambient temperature. Some white fumes were noted to be
evolved. The solution is filtered, and the tan precipitate allowed to dry somewhat. It quickly becomes darker brown, and when added to an amount of
water, it dissolved very slightly, creating a yellow solution. |
I don't recall ever producing blue crystals, though. The sample of tetraaminecopper(II) sulfate monohydrate I have is in the form of a purple
powder. The more gradual addition you attempted may have something to do with that.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Speaking of ligands I have a question.
Say one has a zwitterionic ligand such as an amino carboxylate. Say it is chelating some arbitrary metal ion, silver for example. Is it possible to
protonate the (primary)amine of the ligand with a strong acid? If so would this turn the bidentate ligand to a monodentate ligand? I don't know much
about inorganic chemistry or metalorganic complexes(just started learning about them this week), so pardon my ignorance.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
@Smaerd: This question reminds me of the template approach to macrocyclic ligand synthesis. Great, so you assemble your multi-dentate ligand around
your nice template ion. So how do you free your ligand in order to use it on stuff you want to? One common trick is protonation (of amine based
ligands). The amino groups can then no-longer bind the central metal ion. Another trick is to adjust oxidation state. This can cause change in
coordination geometry and make the macrocycle a poorer ligand for the metal ion (in different oxidation state). I'm sure you can find examples if you
look into maybe templated cryptand synthesis or (I know for certain) there are examples from catenane synthesis (Cu or Au template, IIRC). Rotaxanes
are similarly interesting.
I'm not really sure how (or if) this answers your question, but I thought it kinda relevant. If the carboxylate complex is desired, I'd guess you'd
have to be pretty careful with your acid addition (titration...). I also wonder if the high density of positive charge (from protonating the amino
groups) around a similarly positively charged cation would be a strong enough driving force for the complex to fall apart.
[Edited on 27-11-2012 by DJF90]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
This is really fascinating and might explain some of my experimental results(playing around) from months back  ! Speaking of pH I noticed an insoluble aminocarboxylate bidentate copper ligand upon careful acidification(H2SO4)
became soluble(with no characteristic blue copper colored solution as it was prior to ligand formation). Chelation of an amino acid was initiated by
dissolving Copper Sulfate in an aqueas solution. On basification/neutralization it precipitated again. When it was being dissolved into the acid
solution it made these interesting cracking noises, I had never really heard anything quiet like it in a beaker. In this case, though, I doubt the
ligand fell apart completely. After electrolysis with aluminum cathode and anode a brown precipitate was noted. No further analysis has been done,
yet, maybe I can get one of my professors interested enough to let me have some time with a few analytical instruments. ! Speaking of pH I noticed an insoluble aminocarboxylate bidentate copper ligand upon careful acidification(H2SO4)
became soluble(with no characteristic blue copper colored solution as it was prior to ligand formation). Chelation of an amino acid was initiated by
dissolving Copper Sulfate in an aqueas solution. On basification/neutralization it precipitated again. When it was being dissolved into the acid
solution it made these interesting cracking noises, I had never really heard anything quiet like it in a beaker. In this case, though, I doubt the
ligand fell apart completely. After electrolysis with aluminum cathode and anode a brown precipitate was noted. No further analysis has been done,
yet, maybe I can get one of my professors interested enough to let me have some time with a few analytical instruments.
Rotaxanes are very interesting, spent many day dreams about 'on and off' 'nano-switches' using rotaxanes via electrolysis  . Figured if I could conjure one up maybe it could be bonded or deposited onto a
solid phase or something for logic gates. Again, Day-dreams. . Figured if I could conjure one up maybe it could be bonded or deposited onto a
solid phase or something for logic gates. Again, Day-dreams.
Thanks a lot for the information. It sort of danced around and hit all of my points of interest and left me for some new things to research.
[Edited on 28-11-2012 by smaerd]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Cool, really glad I could help answer your questions and inspire you simultaneously.
Quick question of my own; Its well known that NaBr/NaBrO3/H2SO4 (aq) will yield (chlorine free) bromine, but does anyone have any experience with this
system? My only experience with it was in very dilute solution for a physical chemistry experiment in kinetics; the rate equation for the generation
of bromine by this reaction is actually quite complicated, IIRC, if anyone is interested I can try and find it.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Ethanol can be dehydrated using sulfuric acid to form diethyl ether. This can be further dehydrated into ethylene.
Can dimethyl ether be further dehydrated by concentrated sulfuric acid also? What forms?
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
I don't have a reference offhand, but if memory serves, dimethyl ether will only get protonated to (CH3)2OH+...
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Vanillin can be reduced to vanillyl alcohol with sodium borohydride (Microscale Reactions of Vanillin, Rosemary Fowler, Cottey College, Nevada (see a
link in the references section)), however I lack the latter reagent. Can anyone suggest any other reducing agents? NH4Cl/Zn in acetone,
perhaps?
Any help would be greatly appreciated.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Hexavalent, have you seen this?
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
| Pages:
1
..
19
20
21
22
23
..
30 |