| Pages:
1
2
3
4 |
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Does anyone know at what pressure CO2 gets critical at low temperatures? 73 atmosphere is apperently for ambient temperatures
|
|
|
Vomaturge
Hazard to Others
  
Posts: 286
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
I don't think it can get "critical" unless it is at least 31 C and 73 atmospheres:
https://en.wikipedia.org/wiki/Supercritical_carbon_dioxide
As far as what pressure will liquify it at cooler temperatures, this calculator might help:
http://www.peacesoftware.de/einigewerte/co2_e.html
Bear in mind that below about -56 C, pressure will only solidify CO2, but not liquify it.
Edit: I might add that if there is liquid CO2 present (i.e. a denser water-like phase that settles to the bottom of the pressure chamber), than it's
probably NOT supercritical. This might be more of a technicality, though. Just because CO2 cannot work for a supercritical extraction below
31 C and 73 atmospheres does not mean it is useless. Extractions are done with liquid solvents all the time. Do you have to heat water past 370 C and
20 Mpa to make tea or chicken broth? I would assume that carbon dioxide is a good solvent as a liquid too, and I wouldn't be surprised if nitrous
oxide might work as well. My info about the critical point, triple point, and vapor pressure curve was simply to answer the question of what pressure
to expect at different temperatures. Dense CO2 above 31C=Supercritical. Below -56C=Dry Ice. Anywhere between= liquid, with a vapor space above it, and
a pressure depending on the precise temperature. By the way, you can also make liquid or supercritical carbon dioxide by buying dry ice and putting it
in a sealed container (and heating it if necessary):
https://www.youtube.com/watch?v=-gCTKteN5Y4
I also suppose that if you only had acess to a cylinder of carbon dioxide without a dip tube, you could put the extraction chamber in an ice bath, and
let it condense gaseous CO2 under pressure. A direct transfer of liquid (maybe invert the uncooperative cylinders?) would definately be better though.
Just be carefull to select a container that can easily contain the pressure at your operating temperature; A dry ice bomb full of weed is
doubly illegal some places . .
[Edited on 14-3-2018 by Vomaturge]
[Edited on 14-3-2018 by Vomaturge]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I cant argue one way or other, i doubt your info but equally its something I have seen. I have given every detail i can remember and the most
significant one i was told was to make sure the CO2 tube inside the bottle goes to the bottom.
As the cream whipping stuff, that is pure hearsay and not something i have witnessed.
Apparently there is alot of d-limonene in old car tires!!! I cant remember where i read that but will track it down. The only thing that sticks in
mind was deciding i would only use the stuff i extracted from orange peel!
Thinking about the CO2, freeze drying is done with CO2 in a vacuum isnt it? when you let the pressure back in the stuff evaporates and leaves the
material dry.
So if it takes water straight out of strawberries etc, then why wouldnt it extract from the erb?
Having said all that, there was no vac pump when i saw it done, but its a kind of side thought.
[Edited on 14-3-2018 by NEMO-Chemistry]
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Quote: Originally posted by Mister E  |
Furthermore there is a recrystallizing method referred to as "winterization" using ethanol. I also know this is being done to the extract after the
limonene is removed. |
Winterization is the process used to remove plant fats from the extracted material, the terpenes and canabanoid solubilities aren't too effected by
temperature but filtering while as cold as possible takes out plant fats. Then distil your ethanol back off.
I've heard this makes a product suitable for use in vape pens etc. which would be healthier than mixed combustion products.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Vomaturge  | I don't think it can get "critical" unless it is at least 31 C and 73 atmospheres:
https://en.wikipedia.org/wiki/Supercritical_carbon_dioxide
As far as what pressure will liquify it at cooler temperatures, this calculator might help:
http://www.peacesoftware.de/einigewerte/co2_e.html
Bear in mind that below about -56 C, pressure will only solidify CO2, but not liquify it.
Edit: I might add that if there is liquid CO2 present (i.e. a denser water-like phase that settles to the bottom of the pressure chamber), than it's
probably NOT supercritical. This might be more of a technicality, though. Just because CO2 cannot work for a supercritical extraction below
31 C and 73 atmospheres does not mean it is useless. Extractions are done with liquid solvents all the time. Do you have to heat water past 370 C and
20 Mpa to make tea or chicken broth? I would assume that carbon dioxide is a good solvent as a liquid too, and I wouldn't be surprised if nitrous
oxide might work as well. My info about the critical point, triple point, and vapor pressure curve was simply to answer the question of what pressure
to expect at different temperatures. Dense CO2 above 31C=Supercritical. Below -56C=Dry Ice. Anywhere between= liquid, with a vapor space above it, and
a pressure depending on the precise temperature. By the way, you can also make liquid or supercritical carbon dioxide by buying dry ice and putting it
in a sealed container (and heating it if necessary):
https://www.youtube.com/watch?v=-gCTKteN5Y4
I also suppose that if you only had acess to a cylinder of carbon dioxide without a dip tube, you could put the extraction chamber in an ice bath, and
let it condense gaseous CO2 under pressure. A direct transfer of liquid (maybe invert the uncooperative cylinders?) would definately be better though.
Just be carefull to select a container that can easily contain the pressure at your operating temperature; A dry ice bomb full of weed is
doubly illegal some places . .
[Edited on 14-3-2018 by Vomaturge]
[Edited on 14-3-2018 by Vomaturge] |
Legality has nothing on the trauma you would suffer from the loss of weed at the prices it goes for around here!
Dont laugh but there is a video from ages ago, its about a guy called jimmy (i am not making this up), who went around food factories and then in his
barn tried to replicate things like making rice crispy cereal things. The snap crackle and pop ones...anyway
He did freeze dried peas with a leaf blower and CO2, i also remember a vac chamber he bodged up.
edit as luck has it i found the write up but no video. He did this on TV but it seems the actual video is long gone.
Anyway this is an excerpt from the program blurb
"In this programme Jimmy tries to copy some of the methods food companies use to preserve food to make it safe to eat weeks, months or even
years after it is produced. Why do peas have to be frozen within hours of picking and why is factory freezing quicker than home freezing? Jimmy also
attempts to suck all the water out of strawberries so they can be added to a box of breakfast cereal. Jimmy copies the factory preserving processes
using a leaf blower, a vacuum cleaner, two dog bowls, and the flexible hose from an extractor fan."
http://www.bbc.co.uk/programmes/b00nvg98
shame i cant find the video, from memory considering it was a barn bodge he did pretty good!
Myself i am more dubious of Nitrous Oxide, but it was just something that came up in a chat and not something i have seen.
To clarify i do alot of plant extractions (legal ones) for essential oils for soap making and all sorts of stuff, its mainly what i am interested in,
and was the reason I went to this place to see how they did it with CO2.
As it turns out I havnt ever used it myself, but I have used Butane a good few times to get various plant oils out. For the kind of plants i study its
rarely worth doing with Butane, and nothing gets consumed from the extraction.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
So in theory, this should work?
Use a metal pressure chamber with an over pressure valve. Make a metal rack lifting the plant material to be extracted from the bottom, fill the
chamber with material and dry ice; let it warm up.
Somewhere in the process, the CO2 will become liquid, maybe depend on the pressure, which will extract the material, leaving extract at the bottom of
the chamber?
A tube with a collection chamber at the lowest point of the chamber would be more effective in collecting the extract, and will make the metal rack
unnecessary.
Apparently you would need a pressure of 5.1 atmosphere, where at a temperature above -56 degrees Celsius CO2 will become liquid. Lets say you set the
over pressure valve at 6 atm, and the whole thing should become -56 by itself at some point. 6 atm sounds doable, also in combination with a
collection chamber.
Am I right?
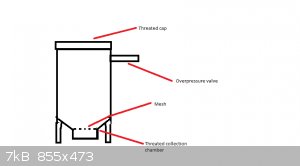
Plus an autoclave/pressure cooker needle valve
[Edited on 14-3-2018 by Tsjerk]
|
|
|
Fulmen
International Hazard
    
Posts: 1749
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
From what I could find with a quick search the solvent properties of CO2 and sCO2 varies with temperature and pressure. Higher temperatures seems to
extract waxes, terpenes and other non-active compounds as well as the primary product, so depending on your goal sCO2 might not be the best option.
It should be possible to build a simple extractor that could extract using both methods. Imagine two chambers (A&B) connected at the top with a
U-bend and a valve. The sample is loaded into the chamber A and filled with liquid CO2. For a subcritical (liquid) extraction one would simply let the
liquid drain into chamber 2, close off and depressurize.
For a supercritical run chamber A would be heated to SC conditions, the extract could then be transferred to chamber B simply by cooling it. This has
the added benefit of eliminating the need for filters since the medium can be transferred with the chambers in the upright position. Adding a third
chamber would allow you to separate out the subcritical-soluble products.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Ok, ok, And how do you suggest obtaining liquid CO2 in this scheme?
|
|
|
Fulmen
International Hazard
    
Posts: 1749
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
You could use something like a Sodastream canister or charge the chamber with dry ice. You could even make a separate tank for liquefying CO2 from dry
ice.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Or you could go over to Airgas and just rent a tank/buy a fill as needed. Be sure to specify a tank with a DIP TUBE, as you want liquid, like someone
making CO2 fog, not a top take off tank for GAS, like a soda or beer dispenser.
And make sure the (occasionally) ignorant dude on the dock at Airgas understands the difference, nothing more embarrassing than a whole stadium worth
of foggers randomly failing to output because a good % of the CO2 tanks shipped to you are either top takeoffs mislabeled as being liquid supply, or
the dip tube came unscrewed/corroded off years ago and is rolling around in the bottom of the tank, but no customer noticed or cared before your crew
rented them.
[Edited on 14-3-2018 by Bert]
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Hmmm, But what would then be the difference between my setup and the two/three chamber setup? Excapt for the fact that there are different chambers?
The liquid , not critical, CO2 would be between around and -56 and -50 (or so) degrees, which would, I guess, minimize secondary extraction of
compounds.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I believe, from the general tone of this discussion, that the home extraction community is headed towards experiencing mechanical explosions due to
jury rigged CO2 extraction chambers implemented by folks with no engineering background to properly spec the parts, in place of the previously
frequent butane vapor explosions and fires...
Pardon my cynicism. I am old enough to remember when hash oil cooks blew up there apartment building PROPERLY- with 95% ethanol and diethyl ether used
in rooms with ordinary, sparking light switches, basements with gas hot water heater pilot lights & etc.
[Edited on 14-3-2018 by Bert]
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I wasn't exactly planning on ending up with stainless steal in my forehead.
I can't weld and am not planning on learning. I'm actually looking for a professional doing this for me as I see a big market for CBD oil, planning to
sell to re-sellers.
My rig would have a needle valve pressure release, and is capable of running completely unattended. Free from any physical manipulation between the
point of loading dry-ice until being back at RT, when back at RT the needle valve will be used to release residual pressure.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Dry ice is a "value added product". They chill it to freezing by boiling off some of the CO2. It costs more per lb. than the liquid, which is also
easier to handle- Pipes. Valves. Pumps. Not moving chunks of dry ice by hand, inefficient of time and labor, dangerous due to frostbite and inevitably
exposing it to atmosphere and FREEZING ATMOSPHERIC MOISTURE ONTO THE DRY ICE SURFACE TO CONTAMINATE YOUR PROCESS, freeze inside your device and block
the pipes, extract the water soluble crap you don't want and generally fuck everything up.
Your entrepreneurial spirit is admirable, but
"A man's got to know his limitations"
Hire a process engineer, or at least an experienced steam fitter.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I think dry ice is easier to handle as with the dry-ice I won't need any pipes/valves/tubing. Just open the lid, dump it in, and wait until it
evaporated. It is also easier to get and transport as I won't need to keep anything pressurized. Only the extractor will be pressurized at some point,
but not at any point during me handling it.
|
|
|
Fulmen
International Hazard
    
Posts: 1749
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Bert: You raise some valid concerns, building the equipment I'm describing isn't for the average amateur. But I know I'm not the only one here with
the skills to do so.
As for the cost, it all depends on your sources. I wouldn't consider dry ice if I could get liquid cheaper, but for a couple of tests the cost of
buying/renting a tank could cost more than a pound of dry ice.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  | I believe, from the general tone of this discussion, that the home extraction community is headed towards experiencing mechanical explosions due to
jury rigged CO2 extraction chambers implemented by folks with no engineering background to properly spec the parts
[Edited on 14-3-2018 by Bert] |
I agree. There is no way you are going to jury rig a supercritical CO2 extraction set-up without creating a bomb.
Search for QWET or QWISO extraction, you will find techniques that give a better product using safer and more food safe materials.
Either way, you will want to experiment with a variety of solvents.
Every extraction that minimizes polar compounds is carried out at low temperatures. Even butane extractions that are done at r.t. (under pressure)
will look like green tar (not gold like you want). Alcohol extractions should be done in sub-zero temps, a little crushed dry ice can help a lot (but
don't use too much!)
Distillation requires a very strong vacuum, under 1mmHg, and a Kugelrohr (or fakelrohr) set-up works best.
The big challenge of extraction is minimizing pick-up of chlorophyll. Chlorophyll is a strange compound; it clumps together and can bind itself to
non-polar and polar substrates quite tenaciously.
Edit: I just want to add that, although cannabis extraction might be legal in your jurisdiction, putting CO2 in sealed containers other than specified
CO2 tanks probably is.
[Edited on 3-14-2018 by happyfooddance]
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I'm also looking for high purity pentane, but I'm still interested in this liquid CO2 thing. We stopped talking about critical CO2 some time ago as
that is indeed not doable at 73 atmosphere. 5-6 atm sounds doable to me though, with a rig welded by a professional.
https://youtu.be/o2aIUemy9Xw
This is in a simple plastic screw top tube
[Edited on 14-3-2018 by Tsjerk]
|
|
|
Fulmen
International Hazard
    
Posts: 1749
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Tsjerk: Regardless of pressure you need a vessel designed for that pressure (plus a generous safety factor). Don't forget that low temperatures can
affect material properties. Fortunately stainless steels tend to have good cold properties, but you still need to factor this into the design.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Thanks for thinking along! I'm thinking about this in design! I'm discussing with a professional welder with experience in pressure equipment as we
speak.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
I would imagine that at only 5-6 atm, your CO2 will be too cold to extract anything. Remember what I said before about adding only a little dry ice to
chill your alcohol to sub-zero temps? Too cold and even alcohols won't dissolve thc. I highly doubt that CO2 would at the temperatures you are talking
about.
If it does it will take a lot of time and multiple extractions. Yields will be poor. More likely than anything I think you will end up with a chunk of
plant matter frozen in a block of dry ice, which you will have to let thaw: which, by the time it does thaw will have condensed lots of water and left
you with green soup.
You are not the first or the five thousandth person to attempt to work with CO2 in this fashion. Best of luck though! I would say to wear your safety
goggles, but I doubt they would do anything to help in the case of mechanical failure of your apparatus.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
OK, we will see what happens. My guess is that -56 degrees Celsius should be enough to get the lighter oils out, which are the aim of the extraction
(I''m aiming for CBD, not THC, although those should behave similarly). The extraction time can be made quite long because it is depending on the heat
input.
I'm not too worried about water condensation as the system is closed, as long as the plant material and dry ice is dry enough it should be fine.
safety; I will put the chamber behind a screen after closing it. and let it run over night. This should give enough time to heat up. I'm still
thinking about a way to release residual pressure remotely.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I'm now working on a simple tube/valve setup made out of 316 stainless.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | OK, we will see what happens. My guess is that -56 degrees Celsius should be enough to get the lighter oils out, which are the aim of the extraction
(I''m aiming for CBD, not THC, although those should behave similarly). The extraction time can be made quite long because it is depending on the heat
input.
I'm not too worried about water condensation as the system is closed, as long as the plant material and dry ice is dry enough it should be fine.
safety; I will put the chamber behind a screen after closing it. and let it run over night. This should give enough time to heat up. I'm still
thinking about a way to release residual pressure remotely. |
Lol.
-56 will get nothing out. CO2 at -30 will get nothing out. CO2 at 0 C° will get nothing out.
Not worried about water? You clearly don't understand what I was saying. You can semi-close the system and if it maintains a slight positive pressure,
you can exclude water. But the point is, a brick of dry ice won't extract anything. You have to evaporate the CO2 and try again. After you try this
and come back and read what I am saying, maybe you will understand.
Safety: placing it behind A SCREEN?!?
You are kidding yourself and heading for hurt.
Residual pressure? It is nothing like "residual". It is more like, increasing rapidly pressure.
Why don't you do a little more research, because many people have tried what you are attempting. The research is less work than constructing an
apparatus which
Quote: Originally posted by Bert  | is headed towards experiencing mechanical explosions
Pardon my cynicism.
[Edited on 14-3-2018 by Bert] |
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Dude? What is your problem? I didn't read all your posts, but the once I did read are all this negative shit like you spill here.
Do you have a clue about what I mean with a screen? Apparently not as you think I'm stupid. I used to make high and low explosives, I know what an
explosion can do, I can perfectly imaging what a steal vessel at high pressure can do, I don't underestimate it. Don't worry.
Residual is relative. My over pressure valve can handle 650 liters of gas per minute. You don't have a clue about the size of
my apparatus, so you don't know how much it will have to handle. Don't make assumptions.
Semi-closed? My apparatus is completely closed with a high over pressure, which you could have known if you would have read what I said and looked at
my ugly drawing. Where is the water coming from?
Did you see the YouTube video I linked too? A brick won't extract no, the idea is to make liquid CO2. It is not going to evaporate before doing so,
and I'm not going to try doing so, thank you for suggesting though.
Do you have references saying cold CO2 doesn't extract? I can't find them, please enlighten me. I like using references when stating something not
commen knowledge, as you can see from my posts in the past.
|
|
|
| Pages:
1
2
3
4 |