| Pages:
1
2 |
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Cursed DHMO! has been known to cause severe burns and is a pollutant so ubiquitous as to have been found in glacial ice!
Holy shit, people, We have an emergency on our hands!
Thanks Franklyn, I needed a laugh today . .
You would figure that in Ca, its degreasing capability would be integrated into some sort of thigh cream as a substitute for liposuction (the burning
let's you know its working); "after the skin grafts my thighs bore no resemblence to those seen before my second child--now only $19.95, etc.".
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
While on the topic, carbon double bonds are amenable to being cleaved
by ozone forming a bridge of parallel ether like and peroxy bonds. This
chlorinated ozonide should be less touchy than hydrogenated variants.
Question is would the explosive decomposition yield phosgene. |
| Quote: | Originally posted by Sauron
What happens with the ethylene ozonide? Does it yield formaldehyde? Because that would be the equivalent scission to what you are proposing (or
speculating about.) |
| Quote: | Originally posted by Ozone
sym-tetrachloroethylene could give 2 equivalents of phosgene, but I envision the production of OCl2, etc. beforehand (and quite likely, explosion). So
on one hand, phosgene (and maybe explosion) on the other, just some formaldehyde and very likely, a comcomitant explosion. Hmm.
|
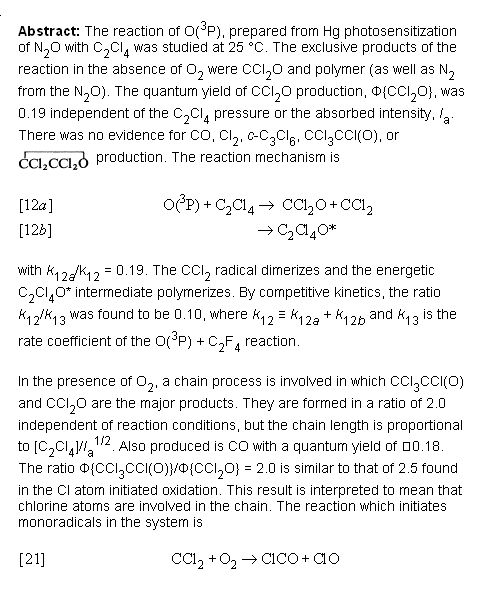
Well apparently it does according to this _
http://pubs.nrc-cnrc.gc.ca/cgi-bin/rp/rp2_abst_e?cjc_v74-579...
Abstract text -
|
|
|
| Pages:
1
2 |
|