| Pages:
1
2
3
4
5 |
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I propose a theoretical synthesis of 2-naphthylisopropylamine:
- sulfonation of naphthalene with conc. H2SO4 at 160- 170°C (at lower temperatures the 1-sulfonic acid is preferred, at higher temps the 2-sulfonic
acid) to naphthalene 2-sulfonic acid
-fusion of potassium 2-naphthalenesulphonate with potassium cyanide to make 2-naphthonitrile (I do not know if this works)
- conversion to 2-naphthaldehyde with SnCl2/HCl:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3...
- then standard reaction with nitroethane and hydrogenation.
Alternatively, 2-naphthaldehyde can also be synthesized by oxidation of 2-methylnaphthalene with selenium dioxide. The synthesis is described in
Organikum.
It would also be possible to start from 2-naphthylamine (how is this made? Nitration of naphthalene produces the 1-isomer afaik) and make the nitrile
via Sandmeyer.
All this would be completely legal.
|
|
|
haribo
Harmless

Posts: 24
Registered: 28-11-2006
Member Is Offline
Mood: No Mood
|
|
64Bandil made and tasted p-F amphetamine. He found it a slightly weaker stimulant than plain amphetamine and slightly 'warmer' so not MDMA-like, but
bending in that direction a little. I've tasted DON and it was unlike DOB or DOC. Still very long-lived but MUCH warmer. Much more like a long-lasting
2CB that a full trip (at 4mg at least). I've often wondered why we've got to things like 4 fluoro thioethyl (2CT21) before trying the nitro fully.
Animal tests can only show so much.
I remember a stimulant that replaced the benzene ring with a cyclopentyl ring. A five member structure would be very interesting from the POV of
adding, say, a methylenedioxy ring.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | BTW would nicodem be so kind to translate that thread from hyperlab? |
http://babelfish.altavista.com/
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
I know this deviates from the spirit of (novel aromatic) substituted isopropylamines, but nicodem had posted some reasearch about
4-nitromethylamphetamine made by nitrating plain 'ol meth and it induced a hallucinogenic type reaction in rats at low doses. it's toxicity is high
but a little lower than methamphetamine (ld50 68 mg) meth is 55 mg. but the ld 50 of mda is 98 mg so its not outright poison I'll bet that has some
potent monamine releasing propertes, nitro fuctions are more electronegative than most on an aromatic ring and that increases receptor affinity.
for something so easily made a human trial would sound interesting enough, those para-substituted analogs have some powerful serotonin agonist action
if you consider 4-methylthioamphetamine causes serotonin syndrome.
us patent 5470997 has an example I can't find nicodem's research on the pharmacology of it.
the example is of a sulfuric acid catalysed reaction using a 2-1 h2so4/hno3 ratio. stir in the cold for one hour followed by quenching and, acid base
work-up to get 60%.
[Edited on 6-12-2006 by jon]
[Edited on 6-12-2006 by jon]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by garage chemist
I propose a theoretical synthesis of 2-naphthylisopropylamine:
- sulfonation of naphthalene with conc. H2SO4 at 160- 170°C (at lower temperatures the 1-sulfonic acid is preferred, at higher temps the 2-sulfonic
acid) to naphthalene 2-sulfonic acid
-fusion of potassium 2-naphthalenesulphonate with potassium cyanide to make 2-naphthonitrile (I do not know if this works)
|
I don't think you would get 2-naphthalenenitrile by fusing potassium 2-naphthylsulfonate with potassium cyanide. The formation of phenols by the
fusion of sulfonates with hydroxides is based on intramolecular oxidation and sulfite elimination, but the cyanide anion is a relatively strong
reducent and thermodynamically the potassium cyanate and potassium 2-naphthylsulfinate would be the preferred products. I'm mostly guessing though, as
I have not checked the literature. (An analogous reaction however works with the sodium salts of the amines)
Friedel-Crafts acylation of naphthalene generally gives a mixture of both isomers but at certain conditions it can yield the thermodynamic product
(substitution at the position 2) as the major product. Here are the two references for the preparation of naphth-2-yl ethyl ketone and a selected one
for the naphth-2-yl methyl ketone:
EP284297 describes the FC reaction of propionyl chloride with naphthalene in dichloroethylene and AlCl3 catalyst. It gives a 2:1 ratio of the
1 vs. 2 substitution.
Zhurnal Organicheskoi Khimii, 16, (1980) 2113-2117 reports only the 2-substituted isomer in the graphical abstract as
the product of the FC reaction of propionyl chloride with naphthalene in chloroform or nitrobenzene and AlCl3 catalyst.
Bulletin de la Societe Chimique de France (1952) 586-592 reports a 75% yield of 2-acetylnaphthalene made with AcCl
and AlCl3. There are also several other references for 2-acetylnaphthalene where this isomer predominates vs. the 1-substituted.
From the naphth-2-yl ethyl ketone (2-propionylnaphthalene) there are so many routes to 1-(naphtha-2-yl)isopropylamine that they are not worth
listing. For those suckers worshiping iodine and red phosphorous the following reference might be interesting:
J. Am. Chem. Soc., 57 (1935) 1091-1093.
Here is the reference for Rothman's first paper in connection to 1-(naphtha-2-yl)isopropylamine:
Rothman et al. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine
self-administration. Journal of Pharmacology and Experimental Therapeutics, 313 (2005), 1361-1369.
It turned out that this compound was moderately researched after all. Here are the other references:
Acta Physiologica Academiae Scientiarum Hungaricae, 3 (1952) 137-151 sounds like a very thorough
classical study to read in regard of this particular compound since its title is "The pharmacological effects of 1-(1-naphthyl)- and
1-(2-naphthyl)-2-aminopropane". A citation from the abstract: " The exchange of the naphthyl for the phenyl group markedly reduced the central
stimulatory activity." This additionally confirms that the compound is not just some boring stimulant, but still does not prove that it is a potential
emphatogen like Rothman's study indicates. I can not get this paper, but would appreciate if someone can.
Arzneimittel-Forschung, 12 (1962) 902-907 reports the compound as inactive in their animal models of CNS stimulant activity.
Tetrahedron, 53 (1997) 4935-4946 describes the synthesis trough the addition of the naphthalene Grignard reagent to a
N-phosphorylated aziridine.
Apparently Glennon did some generalization studies on rats with this compound and reported the results in Pharmacology, Biochemistry and
Behavior, 21 (1984) 895-901. However, I can not get the paper.
GB2122617 claims the electroreduction of 1-(naphth-2-yl)-2-nitropropene to 1-(naphtha-2-yl)isopropylamine with excellent yields (among
several other similar examples).
US2004110791 describes a series of the psychedelic 1-(1,4-dimethoxynaphtha-2-yl)isopropylamines under the pretense of them being "for the
glaucoma treatment" (they certainly do improve vision  ). Among the compounds
there is also described the synthesis and full analysis of 1-(naphtha-2-yl)isopropylamine from naphthalene-2-aldehyde and nitroethane. ). Among the compounds
there is also described the synthesis and full analysis of 1-(naphtha-2-yl)isopropylamine from naphthalene-2-aldehyde and nitroethane.
PS: I know this is completely off the topic of this thread, but then half of the replies in this thread are off topic.
Edit: Added links to patents and corrected a couple of typos.
[Edited on 8-12-2006 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Can someone with the requisite access find those articles mentioned above, and post them here, please? Thanks.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Rothman's JPET papers are freely available, but here are also included most others mentioned:
http://www.4shared.com/dir/1469611/b922a46e/1-_naphth-2-yl_i...
As for the thienylisopropylamines, all the papers are here:
http://www.4shared.com/dir/1469744/b5fdce93/1-_heteroaryl_is...
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Ok, then let me stray even more OT. What about this synthesis route:
Tetralin + HCl + HCHO ----> 3,4-tetramethylenebenzylchloride
3,4-tetramethylenebenzylchloride --(1. Mg, 2. MeCN, 3. NaBH4)--> (3,4-tetramethylenephenyl)-isopropylamine
Two steps, common chemicals.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Unfortunately the resulting 3,4-tetramethyleneamphetamine has a highly unfavorable DA/5-HT release ratio of 53. This is surely not as extreme and
useless as fenfluramine (a completely selective 5-HT releaser) but it is still quite out of the optimal ratio range. However, some find IAP
(1-(indan-5-yl)isopropylamine) interesting even though it has a ratio of 23 (I have no personal experience with it). If taking in account the combined
DA and NE release vs. 5-HT release one gets a similar difference between these two compounds, 18 for 3,4-tetramethyleneamphetamine and 7 for IAP.
Nevertheless, it might be an interesting compound to some degree, if nothing else for combinations with monoamine releasers too rough on the DA&NE
side.
For more information on the compound see:
Nichols et al.
Synthesis and Pharmacological Examination of Benzofuran, Indan, and Tetralin Analogues of 3,4-( Methy1enedioxy)amphetamine.
J. Med. Chem., 36 (1993) 3700-3706.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
Joh posted this comment.
............us patent 5470997 has an example I can't find nicodem's research on the pharmacology of it.
the example is of a sulfuric acid catalysed reaction using a 2-1 h2so4/hno3 ratio. stir in the cold for one hour followed by quenching and, acid base
work-up to get 60%...............
This is the example 6 from the patent.
Example 6
Synthesis of p-Nitro-d-Methamphetamine
Hydrochloride
d-Methamphetamine hydrochloride (4.5g, 2.4x10^-2 mol) was dissolved in sulfuric acid (8.5 ml), and the solution was cooled in an ice-water bath.
Fuming nitric acid (1.4 ml) was added dropwise to the solution. The reaction mixture was stirred at 0 degrees C for 1h. The solution was poured over
ice and 10N sodium hydroxide (35 ml) solution was added to adjust the pH to 12. The aqueous solution was extracted with diethyl ether (3x50 ml). The
combined organic layers were washed with deionized water (2x50 ml), and dried over anhydrous magnesium sulfate. The drying agent was removed by
filtration and hydrogen chloride (1N) in diethyl ether (25 ml) was added to form the hydrochloride salt. The solvent was removed in vacuo. Acetone
(100 ml) was added to the residue and stirred at room temperature for 1 h. The slurry suspension was filtered to give 4.0 g of p-nitromethamphetamine
hydrochloride as a white crystalline solid: mp 192-205 deg C.
What would be the result I wonder of doing this using the 70%
concentrated Nitric substituting fuming nitric acid?
Maybe more H2SO4 could be used to bind the extra water?
Most simple aromatic nitrations like benzene or toluene
for example are carried out using concentrated nitric acid at
room or elevated temperature for some hours reaction time.
[Edited on 6-1-2007 by bio2]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by bio2
............us patent 5470997 has an example I can't find nicodem's research on the pharmacology of it. |
I have not provided the references for the nitration of (N-methyl)amphetamine except for only one which I considered as exemplary (by McBee et al)
simply because they are all more or less the same. Besides the starting material is a controlled substance so it was quite pointless to provide such
references.
However I would appreciate if the discussion about p-nitroamphetamine and it N-methyl analogue would be kept in the thread where they originally started as this thread is getting already annoyingly off topic.
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
OK, sorry, as I thought this was the appropriate thread.
'
If the moderator would please move this then I won't have
to double post.
My question is simply regarding the suitability of concentrated
in lieu of fuming nitric acid in the procedure I posted from
the patent.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Arylethylamine psychotropic recreational drugs: a chemical perspective
Sally Freeman , John F. Alder
Eur. J. Med. Chem. 37 (2002) 527–539
Abstract
The arylethylamines substituted in the aryl ring, side-chain carbons and on the terminal amine, comprise a large number of human mood and behaviour
altering chemicals. Some of these psychotropic drugs have been used since pre-history, but in many states are proscribed and are consequently subject
to clandestine synthesis and illegal traffic world-wide in the forms particularly of amphetamines and to a lesser extent tryptamines. The chemistry
employed in the synthesis of these compounds is dictated often by the available precursors and relies usually on relatively simple, unsophisticated
conversion reactions to a suitable product. The internet web sites and documentation of the recreational drug culture have been studied alongside the
professional scientific and regulatory literature. The review demonstrates the great complexity of the chemistry and neuro-pharmacology of these
chemicals and the challenge faced by legislative bodies to control their traffic and use for the sake of social welfare.
Attachment: Arylethylamine psychotropic recreational drugs- a chemical perspective.pdf (249kB)
This file has been downloaded 1685 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by bio2
My question is simply regarding the suitability of concentrated in lieu of fuming nitric acid in the procedure I posted from the patent.
|
There is no reason to believe the concentrated HNO3 (~65%) would not work (if recalculating the volume of HNO3). No extra H2SO4 is required given that
it is present in more than 6 molar equivalents. Alternatively you can use ammonium, sodium or potassium nitrates instead of HNO3 and you will have
anhydrous conditions (though it is not required). You can even prepare N-methylamphetamine nitrate and slowly add it to sulfuric acid, this way
avoiding the exothermic reaction that follows the addition of the free base in the H2SO4 (it is anyway advisable to add the amine sulfate in the H2SO4
instead of the free amine directly). There are a bunch of possible methods for this nitration.
Not that this is very important but the somewhat wide m.p. of the product in that patent procedure indicates there must some ortho-nitro product
formed. McBee et al report a sharp m.p. of 186-187°C after recrystallization for the para-nitro product and assume that there is always some ortho
product present in the crude products.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Ok, I seem to be unable find Fast & Bulbou's old stimulant chart (can some body post it?) and I must travel to reach scifinder so I'll ask here.
Is anything known about the CNS-effects of diphenyl analog of speed?
Synth of precursor:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0343 (+ refs therein)
Pardon if this has been discussed bee-4...
PS. Don't have the catalogue at the moment, but 2-methylnaphthalene ought to be cheap. How about radical NBS bromination, cyanide swap/hydrolysis (or
make the aldehyde), remaining steps to the final amine are well-documented. Looks like a fun compound. Too bad that G-N hasn't been evaluated as it
would be interesting to compare its effects with those of DMMDA...
[Edited on 11-1-2007 by Sandmeyer]
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
I seem to recall reading about someone's expirience with indanyl amphetamine they said it was'nt very enjoyable.
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Variant
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
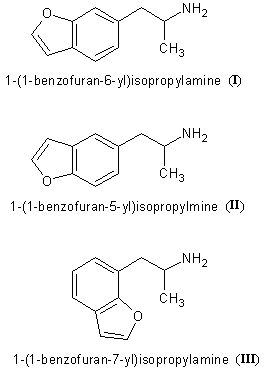
Interesting discussion. Is there anything in the 1-(benzofuran-6-yl)isopropylamine related compounds that has been assayed as psychedelic per se? Or
the thiophene analogs?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I already said this in the first post, but anyway I guess repeating does not hurt too much...
1-(benzofuran-6-yl)isopropylamine and its thio version can not be expected to be psychedelics as they do not fit the SAR for the psychedelics (that is
5-HT2A agonists). They however do fit the SAR of mixed monoamine releasers just perfectly, so they should be expected to be empatogens. The same goes
for the regioisomeric 1-(benzofuran-5-yl)isopropylamine and its thio version, though these should have a less favorable DA/NE/5-HT release ratios
(according to the same SAR theory). I have yet to see a monoamine release/uptake inhibition study for any of these compounds, though their 2,3-dihydro
versions were evaluated by Nichols. But I'll check out if anything else is known on their synthesis, other bioactivity, etc., when time allows.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
interesting articles by Nichols et al.
A.P. Monte, D. Marona-Lewicka, M.M. Lewis, R.B. Mailman, D.B. Wainscott, D.L. Nelson, and D.E. Nichols, "Substituted naphthofurans as hallucinogenic
phenethylamine-ergoline hybrid molecules with unexpected muscarinic antagonist activity," J. Med. Chem., 41, 2134-2145 (1998).
or go to http://www.heffter.org/pages/den.html
and find a whole bunch of relevant articles
[Edited on 5-2-2007 by chemrox]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The only reference for 1-(benzofuran-6-yl)isopropylamine (I) and 1-(benzofuran-5-yl)isopropylamine (II) I could get
is US7045545 (Examples 3 and 1). There are also many others analogues described and lots of interesting chemistry. However, there is nothing on the
pharmacology of these two compounds. All the compounds used in formulations and for which we can therefore suppose the title 5-HT2C agonistic activity
was effective were of the 1-(benzofuran-7-yl)isopropylamine (III) which means the patent is essentially about the 'halfwinged' so
called dragonfly compounds.
Besides this patent I could find no scientific paper about the two compounds. There is something on other potential empatogens like the
1-(benzimidazol-5-yl)isopropylamine, 1-(indol-5-yl)isopropylamine etc., but more about these references some other day.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
That Diphenyl analog looks promising. Stuff like that is where the future of chemistry lies.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
mirapex is a benzothiazole derivitive of amphetamine it's an antiparkinsonian agent that goes directly to d2 receptors. there have been several
lawsuits against it, it apparently works too well and causes compulsive behaviors like gambling and sexual promiscuity in otherwise docile
individuals.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by jon
mirapex is a benzothiazole derivitive of amphetamine |
No, utmost you could call it a cyclic (rotationally restricted) 1-(thiazol-5-yl)isopropylamine derivative if you want to describe it in the context of
this thread:

Pramipexole (Mirapex®)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
Thats an interesting molecule, certainly ive never seen anything quite like it.
|
|
|
| Pages:
1
2
3
4
5 |