| Pages:
1
2
3
4
..
9 |
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I also wanted to have a closer look at the prior step, the one where the base metals are selectively leached.
Let's start with copper's pourbaix diagram in chloride media of course from a thread on SM no less (1)
(1) http://www.sciencemadness.org/talk/viewthread.php?tid=7156
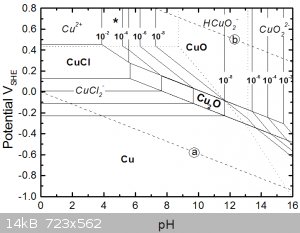
Notice that under acidic conditions copper is most oxidised to copper (II) chloride, but can also form copper (I) when less oxidised.
Copper (I) in concentrated chloride solutions can also form dichlorocuprate(I) anions, CuCl2-, making the copper (I) chloride soluble.
Possibly the copper is acting as the oxidant here and being reduced to copper (I) species, which is then regenerated/oxidised back to copper (II) by
reaction with dissolved oxygen from the bubbling.
Perhaps the peroxide just kick starts things by leaching some initial copper.
Also notice from the diagram that a mixed media of copper (I) and (II) isn't very strongly oxidising, this is maybe the secret to this step's
selectivity, i.e. in not leaching gold. From my first gold pourbaix diagram, the solution needs a minimum potential of 0.8V to be able to even start
to oxidise any gold.
Instead of concentrated hydrochloric acid, here too it might be possible to make a substitution using 'salted vinegar'. Again, it might be kick
started with addition of hydrogen peroxide, but it's the air from the bubbler that regenerates the oxidant and keeps things leaching over until it's
all done.
If air is the key here, one way to improve this is then to improve the mass transfer of air, i.e. have a bubbler that has a diffuser on it to make
very small bubbles so there is a larger surface area for oxygen to diffuse into the water, which is usually the rate-limiting step in such systems.
Pressure is another method of speeding up this mass transfer, but that is extremely dangerous if you don't know what you're doing, so I'm hestitant to
say more about that...
You can always use a combination of bubbling air with large bubbles to keep things thumping about together with a second bubbler with a fine frit to
make diffused bubbles that up's the rate, but doesn't help much with agitation.
[Edited on 14-9-2015 by deltaH]
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Quote: Originally posted by MrHomeScientist  |
Using only household chemicals, and your household fume hood and household acetylene torch  Not quite so easy! Not quite so easy!
A fume hood is definitely required for this. You've got tons of HCl, chlorine, and sulfur dioxide fumes throughout the process. I wouldn't be
comfortable doing this outside, even. It'd be impossible to work next to! Very cool video though, I enjoyed watching it. If I ever get around to
making my fume hood, this is something I'd like to try out. |
I have done this one time in the past when I did not have a fume hood. I did it in my back yard away from people or animals.
If you cover the beaker the sulfur dioxide was not a problem (that I can remember).
I could have used a MAP gas torch (available at the hardware store) to do the melt. It would have taken much longer - 10 to 12 minutes to melt the
gold.
The oxy/acetylene did it in just 4 or 5 minutes. Oxy/acetylene torch set available for about $300 at Harbor Freight.
The chlorine is a different story. I used HCl and bleach to leach some precious metals outside in a bucket before I had my fume hood. I could not
smell the chlorine so I got close and then got a wiff of it - it nearly brought me to my knees. I had to sit down and gather my composure for a few
minutes afterwards.
You are absolutely right - those gasses are nothing to fool with, very dangerous.
kadriver
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
deltaH Golden Fish & Chip Recipe
Seeing as deltaH had a pretty good idea of what do, i agreed to try it out.
In a 250ml beaker, the following were added :-
~6 gold plated fingers cut from RAM boards
1 ancient AMD CPU chip with loads of gold plated pins
200ml 8% white vinegar
10ml 3% H2O2
11.7g table salt NaCl (~1 Molar concentration)
This solution was left to react overnight with air being bubbled through it using an aquariaum pump.
No Airstone was available, so the plastic delivery tube was plugged, and several small holes made in it to spread the bubbles out.
Here are the starting materials and the 'rig' :-
 
The next day it was found that the solution was not really working very well, and had gone a green colour :-
 
An executive decision was taken by High Command to increase the NaCl concentration to 2 [M] so another 11.7g of NaCl was added.
There was no sense in having the CPU chip in the mix, as this step was simply to separate the gold foils from the copper, so the CPU was removed.
This appears to have been more effective :-
 
One strip of fingers appears to be mostly intact, indicating that it was produced by some other process, probably with a different Cu thickness, a
different solder resist mask or something.like that.
All of the above has been conducted at ambient temperatures, currently ranging from a bone-chilling 18 C at night up to around a survivable 28 C
during the day 
The last photo doesn't show the colour well, yet there is a fine green-blue-white crust around the rim of the beaker.
Next step will be to filter out the gold leaves and see if we can dissolve them.
Edit:
At all times it honks of Vinegar without even a hint of chlorine.
[Edited on 15-9-2015 by aga]
[Edited on 16-9-2015 by aga]
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Vinegar, salt and hydrogen peroxide did that? Nice work you guys! I wonder what would happen if the finger that stayed together was allowed to stay
in the bubbled solution for another day? Cool experiment.
kadriver
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Aga, would you mind if I scale up and repeat your experiment using your numbers and make a video of it posted to my YouTube channel?
kadriver
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's deltaH who came up with the idea, not me.
Sure you can make & post a video.
It'd be interesting to see what happens at a larger scale.
A few smaller scale experiments with say 2, 3, 4 [M] NaCl might be a good idea to get a feel for the most effective quantities to use.
Edit:
Maybe call the video 'Step #1' as there is a good chance that deltaH's recipe for dissolving the Gold will work out fine as well.
Further Edit:
Just checked the pot and the foils have started coming off the stubborn strip as well.
[Edited on 16-9-2015 by aga]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I'm also very happy with this. The whole idea is to have people using safer and environmentally more benign reagents when doing this kind of work.
Both he and I would love to see this scaled up!
We would greatly appreciate it if you could link to this thread there so that SM can be promoted. We might also help people with further questions. No
doubt this route will evolve a little more in time.
Aga will be 'out-of-office' for a few days, but the plan was to filter the slurry through a porous filter so as to capture the gold,
but let through the finer milky precipitate (presumable suspensions of copper(I) chloride?). It might be possible to subsequently filter this
copper(I) chloride by using a fine filter paper and then recycle the filtrate liquid as is for the next batch. If so, there's an 'out' for the copper,
which is nice.
In a second step, an attempt to dissolve the gold in a mixture of salt, vinegar and bleach was to be made which you should feel free to do as well.
The recipe numbers are stated above. Be aware that because the solution doesn't start out buffered, some small amount of chlorine might initially be
evolved as a result (or not... we just don't know yet). But even so, it's so much simpler to use vinegar straight without first preparing sodium
acetate, etc. Sodium acetate and the buffer will form anyhow as the oxidation proceeds, so the good news is that the final solution should be buffered
and relatively benign.
Please DO post links here to your videos, we'd love to see more of them!
[Edited on 16-9-2015 by deltaH]
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
So to get started I'm going to scale up by a factor of 10, except for the fingers, I'll use 500 grams of those.
I'll use 2 liters of white distilled vinegar (200 x 10 = 2000ml) from the grocery store.
100ml hydrogen peroxide 3% (10ml x 10 = 100ml) off the shelf at the drug store.
And (11.7 + 11.7 = 23.4g) x 10 = 234g sodium chloride - table salt.
And 500 grams of trimmed circuit card fingers.
All in a 4 liter beaker.
I'll add an air bubbler to provide agitation and O2. Is this O2 required as part of the reaction to lift the gold foils?
Since I'm not a chemist, could you please provide a brief description of the reaction in plain English (for me to include in a text panel in the
video) without acronyms or terms that are too technical?
Thanks for your help! I'm looking forward to this.
kadriver
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Sounds good kadriver. It should take 48h for complete removal, although after 24h many should have separated already.
We don't yet know what the mechanism is for sure. Specific experiments would need to be designed to prove the hypothesis.
That said, my hypothesis is that the peroxide kick-starts things and get's some initial metal salts into solution relatively quickly, but that air is
the net oxidant over most of the run.
In subsequent runs, if you recycle the solution used, you probably don't need the peroxide since the salts that are doing the work are present.
What we're really dying to know is whether the bleached salted vinegar dissolves gold in the next step. It sounds insane, but the Pourbaix diagrams
suggest it should, even if it is a lot slower. I'm holding my breath 
[Edited on 16-9-2015 by deltaH]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
A note on recycling electronics.
Silver and copper are where the money is.
Circuit boards actually contain more of these two
than the average ore extracted from the ground.
But the recycling is only cost effective in bulk.
And you need to recycle your solvents etc to
make it worth while.
They use wood chippers and shred everything
Then process it like ore. They get out a sludge
that is high in rare earths as well. That is further
processed at a different plant where it is mixed
with actual ore.
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
I bought the vinegar and salt (I chose non-iodized sea salt).
I've got everything else.
However I was already into a new video and I am trying to get it published right now - building and operating a sulfuric acid stripping cell to
de-plate the gold off of gold plated items, jewelry.
I'll be able to start this vinegar and salt experiment Thursday or Friday.
I'm kind of sceptacle of the vinegar and bleach dissolving gold - but what a neat discovery that will be if it works!
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
kadriver, I suggest you double the quantities for the bleach reaction just to make sure you have excess reagents to do the
dissolving, but you should add them in two portions, but NB I wouldn't prepare the second portion straight away, because once the bleach is
acidified, it is unstable and will break down into chlorine, so you want it seeing gold at that point, else it will lose its oxidising power .
Add the first, wait for some decently long length of time, then the second. Exactly how long, I cannot say, we shall have to wait and see. Experience
will tell how much excess reagent is required to do the job, but the idea is not to go extreme, that would be wasteful.
My knee-jerk reaction about this dissolving gold is the same, but I'm also trying to have some faith in the theory even when what it is suggesting
sounds so unbelievable.
It's important to understand that it isn't the vinegar dissolving the gold, it's acidified bleach and this is the same as before. This takes gold to
3+ ions which then is quickly complexed by chloride ions, something which gold 3+ has a very strong affinity for. It doesn't matter where the chloride
ions come from, so long as they are present in reasonable proportions, hence why one can simply use table salt. The gold 3+ ions once formed will
quickly bind chloride while the vinegar mops up the rising alkalinity generated by the oxidation to keep things acidic.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I'll be back tomorrow (in Sevilla at the mo) so will get the Bleach & Gold thing started.
Cool ! The race is on !
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
This is so cool! I am hunting for gold to try lol, man if this works you would have solved a big problem waste and cost wise. If I can get the
Hydrogen peroxide (and gold) I will try, I only have 10% acetic acid. I could get normal vinegar but no idea how strong it is, I spose I could boil
some down
[Edited on 17-9-2015 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I based my calculations on 5% acetic acid... so you don't need it strong!
Also, we don't even know if the hydrogen peroxide is completely necessary, it might work even without, just take longer to get going, so maybe you can
test that particular hypothesis for us!
Also, once you have a batch made, you can use the spent solution for the next batch because it already has a whole lot of the metal salts dissolved,
so no kick-starting required... maybe.
aga, oh that's right, your back tomorrow... go aga go aga go, go go, ah...
It would also be good to try it small scale first and work out the bugs so that kadriver can scale up. Not that it would matter much,
because if it fails and doesn't dissolve, he could still dissolve it by other means after a little rinse.
[Edited on 17-9-2015 by deltaH]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I have some old chips with gold bonding wires in and will look for ram chips in a couple of old pc's, ok I will try without the peroxide
Dont ask me, I only know enough to be dangerous
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks, that'd be great. Remember to use 2M NaCl, the 1M didn't work so well for the copper leach step.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Ok as I dont have much containing gold to chuck at this, I thought I would do a couple of tests first with just copper. I figured if the first step
was leaching copper then I could use some thin pcb board with a thing copper coating (the board is new and unused).
I can see what effect it is having on the copper first without gold getting in the way, Its a very thin coating so will cut into strips like the
fingers. The other thing I would like to try with everyone's approval, is using a small electric current. From what I can gather as long as I say keep
to around 0.6V I should be ok?
I have a ATX supply with 3.3V output and a linear technology low drop out regulator, I could use that with a diode in series or even just a few diodes
in series on the 3.3V line. The Voltage wont be a problem as long as I measure the actual drop and watch the temperature, I will run it along side the
gold fingers (when I am happy I have things right) as well. So I could test both with and without electric and see if that does anything?? I intend to
keep the current low ~250mA?
When the time comes to use the gold fingers I could sandwich a finger board between a pcb or even a MMO mesh? that way all the fingers have the Volts
going through them.
Anyone think using electricity is a bad idea? (from a safety point?)
Dont ask me, I only know enough to be dangerous
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Nice ideas, but I don't think applying a voltage to the PCB fingers directly will be practical. While you might get it right by carefully
'sandwitching', these guys want to process 100's g (maybe even kg's) of randomly cut fingers and I strongly doubt you will get them all to make
electrical contact without a lot of effort.
However, you might be able to use electrochemistry in a more generic way, on the solution itself. For example, if an anode could take Cu(I) to copper
Cu(II), then it might be able to replace the role of oxygen. It's probably Cu(II) that's doing the actual leaching of the Cu(0) and then forming
Cu(I). Then on the cathode, you could reduce copper ions in turn to copper metal than can be recovered.
If you can make an MMO basket (like a chips fryer) to hold all your cuttings, this should work, though it's not required to be a basket per say.
This is become a real fish and chips operation! 
[Edited on 18-9-2015 by deltaH]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by deltaH  | Nice ideas, but I don't think applying a voltage to the PCB fingers directly will be practical. While you might get it right by carefully
'sandwitching', these guys want to process 100's g (maybe even kg's) of randomly cut fingers and I strongly doubt you will get them all to make
electrical contact without a lot of effort.
However, you might be able to use electrochemistry in a more generic way, on the solution itself. For example, if an anode could take Cu(I) to copper
Cu(II), then it might be able to replace the role of oxygen. It's probably Cu(II) that's doing the actual leaching of the Cu(0) and then forming
Cu(I). Then on the cathode, you could reduce copper ions in turn to copper metal than can be recovered.
If you can make an MMO basket (like a chips fryer) to hold all your cuttings, this should work, though it's not required to be a basket per say.
This is become a real fish and chips operation! 
[Edited on 18-9-2015 by deltaH] |
Hi.
Yeah guess I didnt think it through  , I will try a basket or something similar
along side the 'normal' Way , I will try a basket or something similar
along side the 'normal' Way  . .
This is what I like best about this place! If something catches peoples minds things get done, its alot like the sodium thread. An idea is first
scoffed at then everyone gets together and starts to experiment  ........One day
Gold from lead might come out of here, I hear they been working on that one for a VERY long time lol ........One day
Gold from lead might come out of here, I hear they been working on that one for a VERY long time lol
[Edited on 18-9-2015 by Little_Ghost_again]
[Edited on 18-9-2015 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Aaaaand it's Gone ! https://www.youtube.com/watch?v=-DT7bX-B1Mg
The Gold foils were vac filtered from the vinegar/salt/H2O2 solution, and then washed from the filter paper into a clean 250ml
beaker as there was other brown gunk on there.
This lost some particles of gold, however it made the results clearler to see. Gentle swirling of the beaker brought most of the gold into a pile in
the middle.

The fingers still had some gold attached although the 'stubborn' one lost all the gold from one side and ~60% from the other. Stirring would have
helped.

The water was brought to approx 66mls and 7g of NaCl table salt was added, and stirred to dissolve.
Then 17ml of some bleach claiming 36g/100ml hypochlorite was added. The liquid measured pH 4.1 on a digital meter at this point.
Immedately a strong chlorine smell was noticed and should have been out in the fume hood. It would have been more useful to add the hypochlorite ml by
ml while stirring.
Within 5 minutes of stirring ALL of the gold foils had dissolved !
Woohoo !

Huge congratulations to deltaH for inventing a NEW gold recovery process ! Well done indeed !
[Edited on 18-9-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Next up is that CPU Chip.

It has been hot-glued to a plastic stir rod and is being twizzled in the solution.

30 minutes in and the pins are showing signs of corrosion, with bare copper clearly seen.
Edit:
Assuming this has all worked out well, how do i get the Gold out ?
Just filter then sprinkle in sodium metabisulphite ?
[Edited on 18-9-2015 by aga]
It's been about an hour now, and not much happening, so another 2g NaCl, ~2ml vinegar and 1ml hypo added.
Seems to have speeded up the leg corrosion.
The stick fell off, so the chip has been wedged in the pot with a bubbler tube.
The liquid is now significantly Brown in colour, reminiscent of a sodium acetate solution.
[Edited on 18-9-2015 by aga]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Wow, amazing work aga! My 'inventing' is pretty useless paper chemistry without friends like you actually getting it done. Thanks
again.
I think for the future then, to minimise the copious amounts of chlorine made, one should definitely prepare the sodium acetate/acetic acid buffer as
stated with the earlier recipe above somewhere, before adding the bleach.
I think it was 70ml 0.2M sodium acetate + 30ml 0.2M acetic acid?
[Edited on 18-9-2015 by deltaH]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
This is some extremely interesting work! Great example of collaboration between the theorists and the experimentalists.
Aga, I would think metabisulfite would work as in the 'usual' recovery process. Or you could cement it out with a more reactive metal (read: any metal
lol).
Did the solution also dissolve the bare copper on your CPU chip, or just the gold? I wonder how selective this process is.
Edit: I wonder where AJ is... bleach and vinegar actually did something. He should be all over this one!
[Edited on 9-18-2015 by MrHomeScientist]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Wooo thats amazing!! Mine is about to be set up  , I have some old circuits
boards (very old modem boards) I will use those and see how selective it is, I might also add some blobs of new solder without the lead in and see how
that does. , I have some old circuits
boards (very old modem boards) I will use those and see how selective it is, I might also add some blobs of new solder without the lead in and see how
that does.
[Edited on 18-9-2015 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
| Pages:
1
2
3
4
..
9 |