| Pages:
1
2
3
4
..
6 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
MSDS for the brand of "boiled" linseed oil sitting in my painting supplies-
http://www.sunnysidecorp.com/pdf/msds42.pdf
Various Cobalt and Manganese compounds are used to catalyze the polymerization, rather than the old fashioned Lead compounds.
As far as de gassing composite fuels before solidification, that may be done with a vacuum chamber and vibration for those formulations which are
fairly liquid and pourable . For mixtures that can be handled like a cookie dough, it is common for amateurs to just consolidate them into motor case
or a surface bonded grain "sleeve" without vacuum or other de gassing techniques. This is popular for smaller motors and less well equipped amateurs-
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Cobalt and manganese compounds would make great burn catalysts to, if they don't cause ignition prematurely during baking.
I'm excited to hear how linseed vulcanisation went. Particularly interested in the MgO 'trick', though I suspect it's not going to be easy to get it
to react. I have a 25kg bag of industrial MgO product (mineral origin). It cost just about nothing, but it's very crude and impure and in the form of
burnt flakes, a bit like burnt lime flakes. I suspect one would need to pulverise this really fine for it to work if my experience with lime
saponification is anything to go on. Then again, 180°C is pretty hot...
To be honest, what I like about this idea (very much) is the low cost and simplicity... which in my opinion is worth having to deal with the smell,
especially if the final set product is not going to be so bad. My factice soap bar I made only smells unpleasant when you stick you nose to it.
So honestly, I don't think it's worth the effort to remove glycerine as I've suggested before. Just need a fume cupboard for the initial stages or
work outdoors far from people and stand upwind  You need to setup an oven outdoors
to bake it during the cure stage... one that you can afford to lose and also never use again for anything else. You need to setup an oven outdoors
to bake it during the cure stage... one that you can afford to lose and also never use again for anything else.
Damn, I really wish I lived somewhere remote and owned a small piece of land... donations welcome of course, all in the name of science 
[Edited on 16-3-2015 by deltaH]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
No significant progress yet, I've spent the afternoon synthesizing MgO. While others might disagree I feel that the smell from a direct vulcanization
is a deal-breaker. Maybe it can work, but I'm not going down that road.
I'll admit that my test was crude, so a more careful setup might mitigate the problem. I'd really like to know how they handle this commercially, but
haven't found anything yet. Nitrogen-flushing is mentioned in the first PDF, but as it is pretty much out of the question for our target marked we'll
have to come up with an alternative.
For now I'm going to explore what MgO can do, but it'll take a couple of days to get a finished product.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I'm glad you're making the MgO, because I think it's going to be key.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
That seems to take it's sweet time. All I could find was some Mg-chips from a band-saw, most likely a mix of pure and alloyed containing ~5% Al and a
tad of Zn. After digesting it in HCl I precipitated it as Mg(OH)2 using NaOH, but this precipitate will not settle out. I'll have to wash/decant it
before drying, so it looks like I need a flocculant.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I don't think "milk of magnesia" will settle out on its own 
You can buy flocculant from the pool section of the supermarket or pool supplier, it's sold as "pool clarifier", in my country. Check composition.
Polymeric anions might work too... e.g. antacid that is based on sodium alginate, e.g. Gaviscon
Also xanthan gum, it's available in some health food retailers here.
Do dilute the flocculant heavily before using it.
I'm happy you're preparing magnesium hydroxide as this is likely the most active form. I don't think bulk MgO would be nearly as reactive.
[Edited on 16-3-2015 by deltaH]
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
You could also try igniting the Mg turnings and collecting the ash (MgO)...
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I tried starch, and while it did seem to help it didn't exactly cause it to settle out. But it thickened enough for filtering through cloth. I'm only
making ~25g, so it's not too much work if one have a bit of patience.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ummm. That's what I would do.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
-- Speculation Alert --
It seems to me that there are some points to be gathered from this:
1. Degree of polymerization, and thus stiffness, is likely controlled by the number of unsaturated bonds in the starting oil.
2. Triglycerides in the starting product may yield glycerol if any strongly acid or base conditions exist. (MgO, H2S) which might lead to a runny,
stinky liquid. (Insert poop joke here)
The following is a table of various oils with their percentage of unsaturated bonds, from Wikipedia:
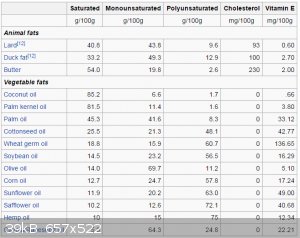
I think a good approach (albeit a few more steps) is to:
1. Choose an oil which gives an appropriate degree of cross-linking when vulcanized
2. Saponify the oil with hydroxide and separate the soap from the glycerin
3. Acidify the soap to recover the unsaturated fatty acids
4. Form ammonia salts with the fatty acids, which dehydrate to amides on gentle heating
5. Add well-mixed oxidizer and sulfur blend to molten amides, cast, and bake to vulcanize.
In theory, the amides will scavenge H2S. The oil industry uses this to desulfurize petroleum. The amides also add nitrogen and hydrogen, which is a
good thing for a propellant. (High delta-Hf for N2, low MW for H2)
Or, use transesterification instead of saponification to make maybe methyl esters of fatty acids, a la biodiesel. Separate the glycerol, add
the oxidizer and sulfur to the oily part, and bake to vulcanize. In theory, the ester falls apart in heat plus H2S acid, and the alcohol flies away on
the wind, which might help with hardening.
If runaway thermal events happen during vulcanization, there is a way around it. Simply pre-vulcanize an amount of propellant in a pan, then shred it
into chunks and mix that with a fresh batch. The pre-vulc stuff reduces the heat generated per unit mass and would probably be effective at stopping a
runaway. This is much like using grog (crushed and fired refractory) to prevent excessive shrinkage in uncured refractory mixes.
In any case, I would advise against leaving the glycerol backbone in any triglyceride intended for use as a propellant. Its abundance of hydroxyl and
C-O bonds do not lend well to energy content or high flame temperature. Glycerol is difficult to burn in general. Have a look at the waste oil burner
industry!
EDIT: Ahh, yes, amides are only very weakly basic. Amines are typical in hydrodesulfurization. Also, one might be trading the sulfurous reek for that
wonderful fishy flavor.
As far as non-caustic and nicely soluble H2S scavengers, try NaNO2.
EDIT EDIT: No, I take that back! Ammonia salts of acids fall apart easily, so start with them. Just skip the conversion step to amides and use as-is.
The conversion will happen during vulcanization and can go two ways: amide formation or entrapment of H2S, forming... err... ammonium hydrosulfide...
Eh, on second thought I think it is going to positively reek no matter what!
EDIT 3: This is highly interesting: http://tenmasub.co.jp/en/products/sulfurchoride-factice.html
[Edited on 17-3-2015 by Praxichys]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I simply have my speculation alert permanently on display as my tag, it's easier that way 
Quote: Originally posted by Praxichys  |
1. Degree of polymerization, and thus stiffness, is likely controlled by the number of unsaturated bonds in the starting oil.
....
The following is a table of various oils with their percentage of unsaturated bonds, from Wikipedia:
|
It's not just about content of polyunsaturated, the type of polyunsaturate is more important. You want an oil that is high in triple
unsaturated fatty acids, not double (both are 'polyunsaturated') in order to have the property of being a decent drying oil and making good factice.
So for example, sunflower oil is very high is polyunsaturates because it is high in linoleic fatty acid derived triglyceride component, a double
unsaturated, but it is not a drying oil and makes crap liquid factice (I made that mistake). Linseed oil is high in linolenic acid derived
triglyceride (~52-55%), which is a triple unsaturated fatty acid, so it does dry to form rubbers and makes good factice. Another even better drying
oil is tung oil, very high in alpha-eleosteric acid derived triglycerides (82%), another tripple double bond fatty acid.
I don't think H2S is the problem, it's probably thiols, the smell is much to terrible to be 'just' H2S. It's more like H2S on steroids 
Removing glycerine may help reduce odour a lot as I suspect it's half of the problem (a la simple thiols...)
PS. Yes the white factice's are nice, but requires sulfur monochloride to make  The whole point of this is to simplify propellants for the amateur.
The whole point of this is to simplify propellants for the amateur.
PPS. On the bright side, the government will love this cause dogs would probably smell it being made from the next city (I'm reffering to the brown
factice) 
[Edited on 17-3-2015 by deltaH]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
There is also the matter of conjugated vs nonconjugated bonds, not sure if that plays a role.
As for saponification, are we even sure that these acids or soaps will vulcanize just as the oils do?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Whole linseeds are also sold by the packet as a health food these days. What about blending the whole linseed and then adding the other ingredients?
All that fiber should stiffen the resulting factice a lot. If you're lucky and can find a bulk supplier of raw whole linseed, it might be fairly cheap
(as opposed to heath food store prices which I find very inflated).
One might fake this effect by making use say blended newspaper and linseed oil for added stiffness. Hell, this is basically the propellant equivalent
of linoleum flooring (made from linseed oil dipped cotton rags) 
The carbohydrate content of the fibre might help the burn.
EDIT: Sorry keep saying linseed, for the whole seed, the name 'flax seed' is more appropriate.
The notion that you could simply blend flax seed, sulphur, potassium nitrate, then compress and bake to make a composite rocket fuel sounds mad
though, I must admit  Warning: blending together may lead to spontaneous
ignition, blend seperately! Warning: blending together may lead to spontaneous
ignition, blend seperately!
[Edited on 17-3-2015 by deltaH]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I think we're far enough off the beaten path as it is 
Good news: The MgO is done, 1 hour @400°C should be enough. I tried heating 10 parts rape seed oil with 1 part MgO, no visible reaction occurred. So
I added 2 parts sulfur and continued heating. The MgO wasn't fine enough to stay in suspension, and the precipitate quickly turned black. I'm assuming
some sulfides are formed. Continuing heating and stirring the oil turned dark brown and thickened within 5-10 minutes. This time the smell isn't as
bad, smells more like elemental or burning sulfur rather than that smell of death I got the other day. Whether it is due to the MgO or more gentle
heating I don't know.
After 30 minutes the factice was still liquid, so I turned off the heat and let it cool. It ended up as a semi-solid sticky goo, not far off from the
previous result. I will try heating it further tomorrow while I dig for the linseed oil.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Sounds very promising Fulmen. I'm VERY pleased to hear that the smell was toned done. I'm excited to hear about your linseed oil
experiments. Best of luck with that!
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
How about this:
A saturated solution of sulfur is prepared in hot xylenes. Some linseed oil is heated to approximately the same temperature. The solid oxidizer is
weighed, finely powdered, and warmed. All three ingredients are rapidly combined to form a dough, which is rammed into propellant cores. Vulcanization
proceeds and the xylene is simultaneously baked out by holding the finished core in an oven until stiff.
The xylene carries the linseed oil and spreads it evenly throughout the oxidizer, much like petroleum ether can be used to spread PiB into RDX.
I did some math on the stoicheometry, and it appears the it may be difficult to get a thorough mix of the oxidizer/fuel binder.
About 92% of rapeseed oil is oleic acid, linoleic acid, and a-linolenic acid, all C18 fatty acids. The average molecular weight of the triglyceide is
expressed as (C3H5)(C18H32O2)3, or C57H99O6, MW
879g/mol.
The combustion of rapeseed oil therefore looks like this:
4 C57H99O6 + 315 O2 --> 228 CO2 + 198 H2O
I have compiled the following oxygen-balanced weight ratios for oxidizer to rapeseed oil:
Ammonium Nitrate - 7.17:1
Potassium Nitrate - 7.17:1
Sodium Nitrate - 6.10:1
Ammonium Perchlorate - 8.43:1
Note that these values ignore any sulfur content, which would cause them to increase.
Also note typical oxidizer to fuel ratios for various compositions:
Black powder, 75/15/10 - 3:1
Zinc/Sulfur - 2:1
APCP: 2.3:1
These do not look stoicheometric. Can ANFO be used as a rocket propellant? The AN version is like ANFO but with vegetable oil and a little sulfur.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I like your out of the box thinking Praxichys, but I fear xylenes could cause the cast to mushroom out during baking due to vapour
bubbles forming and expanding.
Fulmen also stated before that running fuel rich is better and also favours the cast.
[Edited on 17-3-2015 by deltaH]
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Praxichys: Let's not get too carried away just yet. I have real doubts about this endeavor, but it peeked my curiosity so I just want to take a look
at it.
Perhaps we're missing the elephant in the room, the drying oils themselves. Do we really need the sulfur at all, why not accelerate it's natural
polymerization? Yes there are drying additives, but they all depend on oxygen as far as I can tell. Perhaps a peroxide accelerant?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Bad news first: No additional solidification from prolonged heating. So it looks like the edible oils are out. Also I'm unsure if this batch actually
smells any better or I'm just getting accustomed to the smell.
It remelted upon heating and after 30 minutes it was still liquid. So after checking that there were no adverse reactions I added 20 parts KNO3 to the
mix. It formed a dough-like pliable mass that would not sustain combustion when heated with a blow torch.
On the plus side I found the linseed oil, so that will be next on the list.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks for the update, but even from the literature, rapeseed oil didn't look very promising. The rapeseed versions looked like jello. Incidently,
when you said rapeseed, did you mean off-the-shelf Canola oil specifically?
I'm holding thumbs for linseed oil. It would be cool if there was a way to easily separate out useless triglycerides, you know, saturated,
monounsaturated and di-unsaturated. If I'm not mistaken, that makes up ~45% of linseed oil and will only detract from hardness since they can't
vulcanise. I don't suppose simply leaving it in the freezer for a day will do the trick...
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Not Canola, just a no-brand rape seed oil for cooking. Don't know if it matters.
The linseed test is under way, some preliminary observations: First off, the oil is an old bottle of unknown "boiled" quality, fresh or raw might
behave differently.
Heating 10 parts with 1 MgO produced some slight foaming, I'm guessing it's due to water being produced from saponification. This subsided after a few
minutes, producing a darker oil with little change in viscosity.
At this time 2 parts of sulfur was added. Within 10 minutes the mass had thickened to a rubbery dough, more solid than the cold RSO-product. Sadly the
cold product has little strength, crumbling with little resistance when manipulated. I'll let it cool for a few hours and see how it behaves to
reheating.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
From personal experience, flammable oils, either of organic or mineral origin in VERY small percentages will greatly slow a solid fuel or other
pyrotechnic mixture-
This is done deliberately to make "oiled stars" that are intended to smolder all the way back to the ground (or water... I would only fire these over
a body of water, deep snow, etc.). A friend who was less cautious calls these "FSAFOYH" stars (as in "flaming shit falls on your head").
Some will add a % or two of oil in a fast evaporating solvent to black powder rocket fuels that are too fast burning for the intended motor size, then
dry off the solvent. If done correctly, the remaining oil slows the fuel down just enough to prevent a CATO- add just a little bit more, and they
sometimes won't leave the ground!
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I know, it's the most likely explanation for the complete failure of the vulcanized RSO.
As for vulcanized linseed oil, I'm not seeing it. This stuff has no shear strength whatsoever, I see little hope of this working as a reliable binder.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Pppft, that's disappointing. Fulmen are you heating this in a test tube over a flame? Any idea of the temperature?
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
No, I'm using a thermostatically controlled hotplate set at appr. 150°C. One could of course try to optimize the conditions but I think I'll leave
that exercise for someone else.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
| Pages:
1
2
3
4
..
6 |