| Pages:
1
2 |
kjpmi
Harmless

Posts: 13
Registered: 9-11-2014
Member Is Offline
Mood: Chemical-y
|
|
Quote: Originally posted by 420MLGnOhEADsCOPEpro  |
i'd like to buy the chemicals i need but i don't need enough to buy in bulk and otherwise they would cost hundreds or thousands of dollars
i have lots of free time and most of the materials i need so i figure it's reasonable to build something |
I'm going to play devil's advocate here...
When you say the chemicals you need costing hundreds or thousands of dollars, are you refering to the end products? HCl and H2SO4? Because you can buy
each thru many chem supply places online for like $20 to $30 per liter for each. Why would you ever build something like this for end products that
are so cheap?
OR are you refering to the chemicals you need beforehand (Sulfur, Cl gas, etc.)? Everything you need can be found for relatively cheap if you look.
Unless you live in some odd country where they WOULD cost thousands. In that case why not just buy the much more available HCl and H2SO4???? It would
still be cheaper than the precursors.
I understand the fun of making some of the chemicals you need yourself. There are certain things I could easily buy but I preferred doing the
chemistry myself just for the sheer experience.
Then again, I'll just never understand why people go thru quite elaborate, expensive, and convoluted processes only to get shitty yields and product
of questionable quality for some of THE cheapest, most readily available, high grade, already anydrous, etc. chemicals.
Spend the money on glassware or other equipment for the real reactions and syntheses you intend to do, not hundreds or thousands of dollars on HCl and
H2SO4.
Or spend it on other less common chemicals, stock up on a nice diverse lab and try some other obscure, less well known, but really fun and interesting
syntheses.
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kjpmi  |
When you say the chemicals you need costing hundreds or thousands of dollars, are you refering to the end products? HCl and H2SO4? Because you can buy
each thru many chem supply places online for like $20 to $30 per liter for each. Why would you ever build something like this for end products that
are so cheap? |
i don't think you understand
$20/L * 50L = $1000
a 55gal drum full of conc H2SO4 isn't out of the question
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
First $20-30 a liter is WAY more than I would pay for hydrochloric or sulfuric acid. I get sulfuric for about $8 a liter when I buy three at a time,
and hydrochloric is like $6 a gallon at a hardware store.
Anyway the profit margin is huge if you can make sulfuric acid from sulfur, air and water.
You can get sulfur for about $60 for 32 Kg on Alibaba, which is 1000 moles, which in turn can make 1000 moles of sulfuric acid, or about 98 Kg.
If you can sell 98 Kg of sulfuric acid you could easily get $300 if you're practically giving it away. $10.00 a liter and you've turned that $60.00
into $450.00.
It's not at all a bad idea to try to sell it if you can 420pro, or do you actually want to use it all yourself?
[Edited on 23-2-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
It is for me!
In any case you need a proof of concept on a much smaller scale before you build a working prototype before you scale up to industrial volumes. And
at that point the hundreds or thousands of dollars is more a question for your financial backers and your sales pitch.
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  |
In any case you need a proof of concept on a much smaller scale before you build a working prototype before you scale up to industrial volumes. And
at that point the hundreds or thousands of dollars is more a question for your financial backers and your sales pitch. |
this project is not for industrial production as it is definitely more expensive on any scale than SO3 production
the purpose of the project is a stable relatively low cost source of acids that isn't a minimum order of 5 tons
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
selling strong acids requires some level of paperwork as far as i'm aware (for safe shipping i think) and also there's income tax if it's more than
some amount of profit made so i'd prefer to stick to just using it myself
on top of that there are cheaper ways i could make sulfuric acid if i had a larger demand such as
(MgSO4 @ 1124C -> MgO + SO3) if you get 25 tons MgSO4 is about $0.05/Kg and the energy costs ~ $0.02-0.05/Kg = less than $0.13/Kg of H2SO4
and that's not even considering the price of the resulting MgO which i've seen as low as $0.11/Kg so selling for $0.1/Kg that's an extra $0.04/Kg of
H2SO4 in MgO bringing the total to $0.09/Kg of H2SO4 after slightly estimating high (realistic estimate being ~ $0.065/Kg)
it's realistic to sell H2SO4 at >$0.15/Kg bringing profit to >$0.06/Kg of H2SO4 (or realistically >$0.095/Kg, how much greater than depending
on what it sells for)
i may do this at some point considering my current research is high temperature aerogels but any noteworthy production would cost at least $2,000 to
get started likely more than $10,000
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I wouldn't use PVC for sulfuryl chloride. According to this material resistance chart, sulfuryl chloride is incompatible with PVC. I'd use glass for that. 98% sulfuric acid is also not very stable with
the same.
[Edited on 26-2-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Molecular Manipulations  | | I wouldn't use PVC for sulfuryl chloride. According to this material resistance chart, sulfuryl chloride is incompatible with PVC. I'd use glass for that. 98% sulfuric acid is also not very stable with
the same. |
this link doesn't work but a quick google search agrees
i'll just use conduit though because glass pipes are expensive
the pipes shall be soldered with copper (because high temp) for all connections (because conduit doesn't require connectors and costs about the same
it may actually be cheaper than pvc)
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
results part 1
a working apparatus has been made however it strays from the original design
the original downs cell was entirely scrapped after it was found that high temperature gas tight seals are difficult to make without good materials
a membrane cell was then attempted using a piece of paper as the membrane
it was found that the membrane was unnecessary and that HCl worked better than NaCl as a chlorine source
the sulfur burning apparatus was also scrapped as it would require high temperature seals and an alternative was found
sulfur and chlorine react into sulfur dichlroide and disulfur dichlride at room temperature following these equations
S8 + 4Cl2 -> S2Cl2
S8 + 8Cl2 -> SCl2
S2Cl2 + 4Cl2 -> SCl2
this also means that the carbon catalyst is obsolete as the resulting SCl2 and S2Cl2 react with water according to these equations
16SCl2 + 16H2O -> 32HCl + 8SO2 + S8
16S2Cl2 + 16 H2O -> 32HCl + 8SO2 + 3S8
in solution SO2 becomes H2SO3 which reacts with hypochlorous acid according to this equation
H2SO3 + HClO -> H2SO4 + HCl
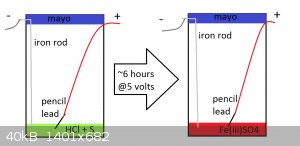
to test this an apparatus was built as such
an old mayonnaise jar was used as the container for the solution
fuming HCl was added to the container
sulfur powder was added to the container
the container was stirred as to distribute the sulfur
a piece of pencil lead and a piece of iron were used as the positive and negative electrodes respectively
a 5 volt DC power supply was acquired
it should be noted that the voltage was not measured but was produced by a phone charger which claims to produce 5 volts
the apparatus was powered for ~6 hours after which the solution had turned red
during the ~6 hours it was observed that the nearby air smelled strongly of chlorine and that the apparatus was fuming the entire time (which hurt my
nose ) )
the red color of the solution is proof that H2SO4 and an oxidiser were produced
tomorrow i will acquire anther piece of pencil lead to replace the iron electrode which will prevent the H2SO4 from being consumed (unless it rains
all day)
i will also take some pictures
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
i forgot to mention that the method i used will not work with NaCl instead of HCl
if you use NaCl the Na will form NaOH which will then react with the sulfur into polysulfides and thiosulfides and possibly other things thus not
getting you sulfuric acid or hydrochloric acid
luckily this is easily avoided by electrolysing the NaCl in a separate container and then venting the chlorine to the sulfur and water mix
it may be beneficial to have the sulfur and water in different containers as well (at the very least the end product doesn't have to be filtered)
though this can cause problems
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
results part 2 (pics)
today i acquired another carbon electrode and began another test
i could not produce enough sulfuric acid to make a copper sulfate solution because i had the apparatus set up wrong for most of today but i should
have some tomorrow
anyways here are the pictures i took today
1st pic is the iron electrode that was used
yesterday it was green from the chlorine but somehow it rusted overnight
2nd pic is yesterdays solution with the sulfur filtered out
it was red orange before i filtered it and when i began filtering it
i used extra water to get more of the salt through the filter but upon diluting it became yellow (is this from ph change)
i would have taken a picture before if i knew this would happen
i'm currently letting some dry out in a dish but it's rather humid today
3rd pic is residue from some of the solution i spilled
when spilled it reacted with the concrete because there's still a notable concentration of acid in it
the red color is definitely the same that i saw in solution before it was diluted
4th pic is the apparatus running the second batch
5th pic is inside the apparatus while it's running
the contents of the jar are yellow from the sulfur dust
the middle of the jar is lighter because there is sulfur on the surface there whereas the rest of the sulfur has sunk (i found that the entire surface
of the water should be covered in sulfur for the apparatus to work efficiently after i noted there was very little sulfuric acid in solution and lots
of escaping chlorine)
the dark rod in the bottom of the container is an electrode that fell in during setup
the dark ball in the bottom of the container is a wad of copper wire that i used as a test for sulfuric acid (as sulfuric acid reacts with copper to
make blue copper sulfate and hydrochloric acid does not react with copper(chlorine reacts as well but it makes green copper chloride and that reacts
with sulfuric acid into copper sulfate as well))
so there you have it
there should be a few more pictures tomorrow too
    
|
|
|
| Pages:
1
2 |