| Pages:
1
2 |
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Anyway, I did send him a list of other criticisms. There has been no reply.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's too bad. When was the paper written?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
The publication date is April 2014.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Then he should be responding. That's really bad...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Oh, look- an even worse paper has been accepted for publication!
http://scholarlyoa.com/2014/11/20/bogus-journal-accepts-prof...
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Yep, that's definitely a peer reviewed journal I would consider it a mark against
me if I had a paper in a journal like that. I would consider it a mark against
me if I had a paper in a journal like that.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
That one is a perfectly good paper !
Accurate in every respect.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
I actually found a worse paper.....
http://www.ijpsi.org/Papers/Vol3(6)/B036105011.pdf
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2793
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
We have discovered that bacteria are inhibited by shitloads of copper! 
|
|
|
KoiosPhoebus
Hazard to Self
 
Posts: 53
Registered: 23-1-2023
Member Is Offline
|
|
I read the text before the paper, saw "complexes" and went "Oh NO". It's an
extremely common mistake for scientists, especially biologists (note: I'm a microbiologist/geneticist myself, so this is not me throwing shade at
another department), who are unfamiliar with the intricacies of the field. If you look into making metal complexes, you'll find some good procedures
amongst a heap of "the equation balances so it works!  ". ".
My favourites are the ones who react metal sulphates with a very weak acid, most commonly an amino acid like glycine, and then claim they got the
chelate of the weak acid plus sulphuric acid as the side-product. Example below:
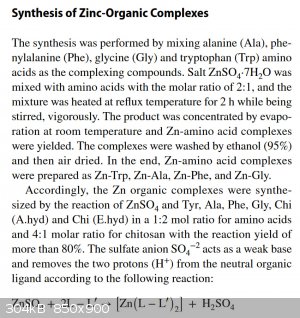
(another error - they refer to tryptophan (Trp) in their first para, but in their second para they refer to tyrosine (Tyr), a different amino acid)
This is frustratingly common in horticultural/agricultural research. I recall a paper which attempted to synthesise ferrous "amino acid chelates" by
directly reacting FeSO4 with either glycine, arginine, or histidine. The problem is that the amino groups in all three amino acids would be
protonated at pH<9, so they actually obtained adducts where Gly/Arg complexes the iron through monodentate coordination of the carboxylate group to
the metal (complex II in the below).

Source: https://doi.org/10.1021/jp054119b
(Histidine is slightly different as it can coordinate to metals through the imidazole ring, and hence can form bidentate chelates even as a neutral
zwitterion. However, it would still be an adduct instead of a chelate e.g. ferrous bis-histidinate.)
Stuff like this matters because the adducts are significantly weaker complexes; glycine, for example, has a complex stability constant of
K=101.09 for its ML complex with calcium but a complex stability constant of just K=100.3 under the same temperature + ionic
conditions for its protonated M(HL) complex (stability constant calculated as [MHL]/[M][HL]). With iron(III), the same constants are
K=109.25 for the fully-deprotonated ML, versus K=103.7 for M(HL). If anyone's interested in the stability constants of various
complexes, someone has made a database explorer for the old NIST stability constant database which has unfortunately been discontinued - this is where I got
the above figures.
This even shows up in biological assays; as an example, one study found that ferrous glycine sulphate (the adduct) has a significantly higher rate of adverse events than ferrous bisglycinate (chelate).
Ever since "chelation" became the trendy thing to do to mineral nutrients to make them more available, there's been a whole range of papers by people
employing methods which don't stand up to scrutiny to make their "chelates". I should add that I have attempted to and failed to replicate many such
methods, because many of them are simple enough that if they did work, they would make metal complex synthesis so much easier.
As an example, this paper claims to have synthesised histidine complexes (with no chloride) by directly reacting the metal chloride with histidine in an aqueous
methanol solution. This would be such an easy synthesis method if it worked! Unfortunately, histidine has low solubility in water, and L-histidine has essentially no solubility in methanol (plus, methanol is actually the worst solvent for the stereoisomer D-histidine), so I have no idea if the histidine would even dissolve into the
solution. Plus, there's the classic issue of "where does the anion (chloride, in this case) go? The conjugate acid of the anion is too strong...".
Nevertheless, I decided to try and replicate the procedure using manganese chloride, and even after 4 hours of reflux, the primary precipitate was
undissolved L-histidine (the L-His I used came in needle-shaped crystals, so I was able to identify its presence in the precipitate). I gave up after
about the 5th hour and left the manganese chloride/L-histidine/methanol/water mixture in a tube on a shaker for about a month. When I came back, there
was a little bit of light brown precipitate, but most of the L-His crystals remained undissolved, at which point I gave up and tossed the entire tube
out.
The procedure didn't make sense theoretically, I spent a little L-His to give it a fair shot anyway, and it didn't work despite me giving it
reasonably favourable conditions for success. In my experience, that's how all of these have gone.
|
|
|
| Pages:
1
2 |