| Pages:
1
2 |
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
I've tried to condense potassium sulfamate with glyoxal, in many different conditions, no hexasulfamatoisowurzitane was formed or the yield is
neglible, instead tetrahydroxodisulfamatopyperazine is obtained in almost quantative yeild.
I got new idea about how CL-20 can be made. Book "Organic Chemistry of Explosives" at page 278 mentiones that TNGU is easily hydrolised to
tetranitraminoethane. That can be good precursor to HNIW, idea is to condense tetrahydroxodisulfamatopyperazine with tetranitraminoethane, this should
result in formation of isowurzitane cage with 4 nitrogroups already placed, with 2 sulfamate reminants witch can be easily nitrated to HNIW.
Scheme of reactions, i propose is shown below:

[Edited on 22-8-2008 by Engager]
|
|
|
Axt
National Hazard
   
Posts: 796
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Heres an abstract, though they dont give much away.
Synthesis and analysis of N,N',N'',N'''-tetranitro-1,1,2,2-ethanetetramine and energetic salts thereof. Lee, YiewWanq; Goede, Patrick; Latypov,
Nikolaj; Oestmark, Henric. DSO National Laboratories, Singapore, Singapore. International Annual Conference of ICT (2005), 36th(Energetic
Materials), 124/1-124/9. Publisher: Fraunhofer-Institut fuer Chemische Technologie, CODEN: IACIEQ ISSN: 0722-4087. Journal written in English.
CAN 144:372481 AN 2005:824848 CAPLUS
Abstract
Conference proceedings. One of the most interesting energetic mols. developed in recent years is the highly energetic polycyclic nitramine,
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20). Due to difficulty in synthesis, the price of this material is very high. Hence,
there is a need for an improved method of prepn. The crucial cage formation was envisioned to proceed by condensation of
N,N',N'',N'''-tetranitro-1,1,2,2-ethanetetramine (TNAE) with 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine followed by nitration to produce CL-20. In
this work, TNAE was prepd. by hydrolysis of tetranitroglycoluril and isolated as the free base. TNAE was converted to the corresponding tetra salts
with ammonium (ATNAE), potassium (KTNAE) or guanidinium (GTNAE) as counter ions. TNAE and its salts were characterized and found to be highly
energetic with reasonable to low sensitivity which makes them interesting as new energetic ingredients.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Axt
Heres an abstract, though they dont give much away.
Synthesis and analysis of N,N',N'',N'''-tetranitro-1,1,2,2-ethanetetramine and energetic salts thereof. Lee, YiewWanq; Goede, Patrick; Latypov,
Nikolaj; Oestmark, Henric. DSO National Laboratories, Singapore, Singapore. International Annual Conference of ICT (2005), 36th(Energetic
Materials), 124/1-124/9. Publisher: Fraunhofer-Institut fuer Chemische Technologie, CODEN: IACIEQ ISSN: 0722-4087. Journal written in English.
CAN 144:372481 AN 2005:824848 CAPLUS
Abstract
Conference proceedings. One of the most interesting energetic mols. developed in recent years is the highly energetic polycyclic nitramine,
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20). Due to difficulty in synthesis, the price of this material is very high. Hence,
there is a need for an improved method of prepn. The crucial cage formation was envisioned to proceed by condensation of
N,N',N'',N'''-tetranitro-1,1,2,2-ethanetetramine (TNAE) with 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine followed by nitration to produce CL-20. In
this work, TNAE was prepd. by hydrolysis of tetranitroglycoluril and isolated as the free base. TNAE was converted to the corresponding tetra salts
with ammonium (ATNAE), potassium (KTNAE) or guanidinium (GTNAE) as counter ions. TNAE and its salts were characterized and found to be highly
energetic with reasonable to low sensitivity which makes them interesting as new energetic ingredients. |
Can you get this reference? I have both 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine and TNAE prepared, witch are conditions for mentioned
condensation?
|
|
|
Axt
National Hazard
   
Posts: 796
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
No I dont have access to the article, though i suspect that it doesn't give the conditions of the condensation. The fact that it wasn't mentioned in
the title/abstract either suggests that it was a failure or a just preliminary investigation of the precursor TNAE.
I may be wrong or it may hold other leads to the reaction, if your desperate enough it is for sale online:
http://www.ict.fraunhofer.de/EN/VuM/Proceedings/index.jsp
For the prep of TNAE, is it as simple as reacting 1 mol TNGU with 4 mol NaOH and precipitating with HCl?
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Axt
No I dont have access to the article, though i suspect that it doesn't give the conditions of the condensation. The fact that it wasn't mentioned in
the title/abstract either suggests that it was a failure or a just preliminary investigation of the precursor TNAE.
I may be wrong or it may hold other leads to the reaction, if your desperate enough it is for sale online:
http://www.ict.fraunhofer.de/EN/VuM/Proceedings/index.jsp
For the prep of TNAE, is it as simple as reacting 1 mol TNGU with 4 mol NaOH and precipitating with HCl? |
Yes, but TNAE is soluble and not precipitates as easy as you writen, TNGU hydrolizes in alkali envouriment almost permanently (like sodium carbonate
with acid), in neutral conditions conversion cal also be completed but takes longer time.
|
|
|
Peroxid
Hazard to Self
 
Posts: 56
Registered: 23-8-2006
Member Is Offline
Mood: No Mood
|
|
Hi!
Have somebody success with synth of TADB ( from HBIW (the usual way to reductive debenzylating)?
I did many experiments to synth TADB, but i haven't succes with it, i always got a brown oil :/
Any idea? Thanks in advance!
|
|
|
ksj_6808
Harmless

Posts: 20
Registered: 6-7-2012
Member Is Offline
Mood: No Mood
|
|
40% Glyoxal can be bought at www.elementalscientific.net.
And you dont have to be a company, anyone can buy there!!
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Engager  | I've tried to condense potassium sulfamate with glyoxal, in many different conditions, no hexasulfamatoisowurzitane was formed or the yield is
neglible, instead tetrahydroxodisulfamatopyperazine is obtained in almost quantative yeild.
|
here is the link: http://www.pirotek.info/VV/HNIW_CL20_.html . If you have problems reading russian text, I translate it. 9.71г sulphamic acid mixed with 5 gr
H2O. Slowly added 5.6 gr KOH dissolved in 6 gr H2O , cooling this mixture, than added few drops of solution of KOH to make pH slightly basic. Some
additional water to dissolve all kalium sulphamate, then a little bit of sulphamic acid to make pH= 3-5 . Then added 4.84 gr 40% solution of glyoxal
during 60 min. Stirred mixture for 3 ours, than waited for 40 ours (may be you wanted to get desirable compound too quickly?). Filtered precipitate
weigh 6.2 gr. Then nitration- 3ml 98% HNO3 + 0.5 ml 96% H2SO4. Stirred for 2 ours cooling mixture by means of ice bath, then 4 ours at room
temperature. Then put all into crushed ice.
Women are more perilous sometimes, than any hi explosive.
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Sorry, small correction. Then nitration- NOT ALL AMOUNT, OBTAINED AT THE PREVIOUS STAGE BUT ONLY 0.8 GR OF IT is to be placed into mixed acid- 3ml
98% HNO3 + 0.5 ml 96% H2SO4. Stirred for 2 ours cooling mixture by means of ice bath, then 4 ours at room temperature.
Women are more perilous sometimes, than any hi explosive.
|
|
|
detonator
Harmless

Posts: 29
Registered: 12-4-2011
Location: Maoming City, Guangdong Province, China.
Member Is Offline
Mood: No Mood
|
|
I think that the most troublesome is the hydrogenolysis step.
I've always wanted to seek a way to omit this step.
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
I found some info about condensation of potassium sulfamate with glyoxal. One russian book, here is the reaction's scheme:

There are three sorts of explosives, which can be obtained in that way. Bad news is that the most interesting compound (92) has very small yield. (90)
is the well-known TEX, (91) is the powerful explosive (not as powerful, as CL-20), but interesting too.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I think that TNGU is very very powerful itself 
Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Here is some information about Hexanitrohexaazaisowurtzitane, which is sometimes referred to using the acronym HNIW, or by the code-name Cl-20:
Heat of formation (observed): +228 cal/g
Detonation Velocity: 9.38 km/sec
Density: 1.98 g/cm3
Calculated detonation pressure: 428 kbar
The alpha crystal structure consists of rhombic prisms, while the beta consists of either colorless needles or chunky prisms.
Impact sensitivity: using 2.5 kg "hammer" to measure the drop height required to produce a 50% probability of detonation, the alpha form gave an
average value of 17cm, while the beta form gave a value of 21cm. Compare this with a value between 23 and 25 cm for HMX.
A plasticized composition of 90% HNIW and a 10% mix of HTPB (used as solid rocket binder) and PL1 (plasticizer which is a 1:1 ratio copolymer of
poly(3-butyl-co-3,4-dibutylthiophene and 3-butyl and 3,4-dibutyl thiophene) is recommended since Cl-20 is somewhat more sensitive than HMX. This
composition has measured VOD of 7.83 km/s, with calculated pressure of 330 kbar. So while HNIW seems to be powerful in its pure form, this pure
compound is not very practical. The safer and easier to handle plasticized/castable composition of HNIW does not have extremely impressive
performance. There are several other highly energetic and more insensitive compounds that require less plasticizer and binder to be both tolerably
resistant to impact and have desirable moldable/castable properties. Another thing to consider is that HNIW in compositions is significantly less
dense (1.82 g/cm3) than the pure compound, whereas the other nitramines do not lose as much density when formed into compositions.
Synthesis of the Precursor:
HBIW is one of the precursors to HNIW, and basically has the same molecular shape and structure of HNIW except that the 6 nitro groups are all
replaced by benzyl groups (basically just toluene molecules connected on their methyl groups).
The procedure for making HBIW (2,4,6,8,10,12-Hexabenzyl-2 ,4,6,8,10,12-hexaaza-tetracyklo [5.5.0.0.0] dodecane) stems from J. Org.Chem. (1990),
55, 1459. (1990), 55, 1459th.
To verify only a slight adjustment of preparation was made.
O=CH-CH=O ...... formic acid, CH3CN
........................... ---------------------------> HBIW
C6H5-CH2-NH2
A. From left to right in the picture: formic acid of 80-90% concentration, acetonitrile as solvent, 40% aqueous solution of glyoxal, and bezylamine:

B. The two-liter Erlenmeyer flask will present one liter of acetonitrile, 100 ml, 120 ml of benzylamine (117.9 g, 1.1 mol) and 4.8 ml 80-90% formic
acid (5.76 g, 0.11 mol). Flask placed in a bath of ice and water and stir (preferably with a magnetic stirrer). Wait until the temperature drops below
15 ° C.
(picture on the right below)
C. To the solution slowly, stirring constantly, add Glyoxal 40% (72.5 g, 57 ml, 0.5 mol). The whole procedure should take about an hour. Adding
either through a dropping funnel or better using a peristaltic pump. The temperature should not exceed 20°C. Shortly after beginning the addition of
glyoxal in a solution of the product begins to appear as a white crystalline cavities, (as shown in the picture on the left). When introduced into
all the glyoxal reaction, remove the cooling bath and the solution for about 30 minutes stir.

D, then remove the stirrer, cover the top of the flask with aluminum foil, and place it in a dark place. Leave it at room temperature for 24 hours.
During this time, will slowly change color from white to a mixture of yellow and orange:

E. Finally, crystals of the product are filtered and twice rinsed with acetonitrile. The filtrate can strip aqueous acetonitrile and use it for the
next reaction. In this case, has distilled all the liquid, but only 80-90% of the volume.
The solvent thus obtained can be used for further condensation, it only add acetonitrile to volume of 1100 ml. Do not add water.

F. The product can be obtained by being recrystallized in acetonitrile, and can then be used without any purification for the next reaction. The
product from this procedure should be around 90 g (0.12 mol, 76%) of HBIW, you can see the final picture below. HBIW is a slightly yellowish white
solid with a melting point of 155-157 °C.

[Edited on 1-11-2012 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
SyphonNexus
Harmless

Posts: 3
Registered: 18-1-2013
Member Is Offline
Mood: No Mood
|
|
Recrystalization of CL-20
Here is another possible recrytalization method that yields a more impact insensitive HNIW. More insensitive then RDX and HMX. Though it only hints at
it in in the pdf as I see it, but I'm inexperienced and still learning maybe y'all can make better use of this info.
www.wydawnictwa.ipo.waw.pl/cejem/Cejem-8-3.../Elbeih.pdf
EDIT: Copy and paste link to a search engine. The PDF file should be the first result.
[Edited on 19-1-2013 by SyphonNexus]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Crystallization & Characterization of RDX HMX & CL-20
www.researchgate.net/publication/231229915_Crystallization_and_Characterization_of_RDX_HMX_and_CL-20/file/79e4150b73a605f438.pdf
CL-20 Sensitivity Round Robin
www.dtic.mil/dtic/tr/fulltext/u2/a415096.pdf
.
|
|
|
SyphonNexus
Harmless

Posts: 3
Registered: 18-1-2013
Member Is Offline
Mood: No Mood
|
|
Does the geometry of the crystals also play a part in its shock sensitivity? I know the structure of the chemical it-self plays a dominate role.
EDIT: Never mind I found my answer in the "Crystallization & Characterization of RDX HMX & CL-20" PDF.
[Edited on 19-1-2013 by SyphonNexus]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
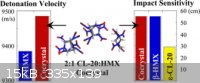
Researchers recently made a cocrystalline form of HNIW with HMX. It has the same sensitivity as HMX but has a detonation velocity 100 m/s higher. The
crystal consists of HNIW and HMX in a 2 to 1 molar ratio, respectively.
"High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL-20:HMX" Cryst. Growth Des., 2012, 12 (9), pp 4311–4314
Seems like a practical incremental improvement.
[Edited on 22-1-2013 by AndersHoveland]
|
|
|
SyphonNexus
Harmless

Posts: 3
Registered: 18-1-2013
Member Is Offline
Mood: No Mood
|
|
Isn't the power of an energetic material the square of its VoD? If so isn't 100 m/s a nice improvement?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Yes, a small increase in velocity does represent a greater increase in explosive force, especially for velocities that are already high. But this 100
m/s increase actually only represents a 2.2% increase in explosive power here.
Not a big ammount, but the extra expense for this small improvement in explosive performance can still be economically justified in a military weapons
delivery system with a high cost, as the price of the explosive charge may only represent a tiny fraction of the overall cost of the weapon. During
the Gulf War, one American military advisor described it this way: "We are using a 1.2 million dollar cruise missile to carry what basically ammounts
to just a hand grenade. Whoever ordered that attack should be court marshalled."
Also to mention, the strategy of cocrystallization is never going to lead to any huge breakthroughs, as crystallization is more of a physical
phenomena, and the molecar composition is by far a much greater factor.
[Edited on 22-1-2013 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
attached is information comparing sensitivity of pure Cl-20 to its lower sensitivity co-crystal with HMX.
|
|
|
| Pages:
1
2 |
|