| Pages:
1
2
3 |
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fiberdrunk  |
I wonder if the ink precipitated out when they added the egg shells? The calligraphy website IAMPETH has a .pdf article for "freshening" an iron gall once it's separated out, by adding vinegar. The acidity keeps the iron from dropping out.
|
Maybe you just don't need as much acidity. Vinegar is already a 1:20 dilution of acetic acid with water, so if adding 4 drops vinegar to what I'm
guessing is 1 oz. of ink is enough to re-dissolve the iron, then maybe you don't have to have a nib-eating acidity.
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Whoa dude ! No Insult meant, implied or otherwise.
Apologies for the unintended offence.
Just seemed Mad (to me) to be boiling gallons of acorns for two days.
Which Utterly Insane person first did that, and were they burned at the stake for doing so ?
When it was first done, it is HIGHLY unlikely that it was seen as a sane thing to be doing.
[Edited on 6-7-2014 by aga] |
Lump me in with the insane... I processed 15 gallons (dry weight) worth of black walnuts into ink last September/October, resulting in over 5 gallons
of wonderful finished ink.
I processed 5 of these bucketfuls:


Sure, you can buy bottled ink. But some of us find the process fascinating and even enjoy it!
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Artemus Gordon  | Quote: Originally posted by fiberdrunk  |
I wonder if the ink precipitated out when they added the egg shells? The calligraphy website IAMPETH has a .pdf article for "freshening" an iron gall once it's separated out, by adding vinegar. The acidity keeps the iron from dropping out.
|
Maybe you just don't need as much acidity. Vinegar is already a 1:20 dilution of acetic acid with water, so if adding 4 drops vinegar to what I'm
guessing is 1 oz. of ink is enough to re-dissolve the iron, then maybe you don't have to have a nib-eating acidity. |
It would be fascinating to push the envelope, to see just how little acidity I could get away with...
I've really been enjoying this forum and the resources here, even if I'm not a chemist. I enjoy seeing what people are trying to achieve in their
experiments. You chemists are so cool! I'm realizing iron gall ink may very well be my gateway drug into the much bigger world of chemistry...
[Edited on 9-7-2014 by fiberdrunk]
[Edited on 9-7-2014 by fiberdrunk]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cool !
Making is Definitely much more fun than just Buy.
Never seen a black walnut fruit pod thing ever before.
What is the warning sign on the bucket ?
Looks like a 3 armed infant attacking a cup of tea.
Excellent photos.
| Quote: | | . I processed 15 gallons (dry weight) worth of black walnuts |
What was the 'process' ?
[Edited on 9-7-2014 by aga]
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Cool !
Making is Definitely much more fun than just Buy.
Never seen a black walnut fruit pod thing ever before.
What is the warning sign on the bucket ?
Looks like a 3 armed infant attacking a cup of tea.
Excellent photos.
| Quote: | | . I processed 15 gallons (dry weight) worth of black walnuts |
What was the 'process' ?
[Edited on 9-7-2014 by aga] |
I guess the warning label on the bucket was a drowning warning for infants! Normally people use these buckets for paint.
You can see the black walnut ink-making process here.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Fascinating.
"boil it covered for 10 minutes to kill the ink beasties"
What beasties ? Bacteria, Virii ?
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Fascinating.
"boil it covered for 10 minutes to kill the ink beasties"
What beasties ? Bacteria, Virii ? |
The fermentation. The ink is full of biological activity while it's fermenting. If you don't boil it, it would most likely mold over.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Micro organisms. Phew.
I had visions of the man-eating Ink Beasties leaping from manuscripts everywhere and taking over the world.
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Ok, so I'm getting a little bit concerned, because I have my oak galls fermenting in a sealed glass jar with water (they've only been been in there
about a week), and it's already been so active that the lid of the jar is pressurized outward. Is this something to worry about? Do I need to let off
the pressure, or will it settle down?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It'll be CO2, so let it out.
Unlikely to allow world-eating aliens out.
Best check with an experienced Ink Pressure Daemon Master first though.
Edit: Ideal Gas Law
Ideally, Gas Pressure should be controlled to prevent explosions.
[Edited on 9-7-2014 by aga]
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | | Ok, so I'm getting a little bit concerned, because I have my oak galls fermenting in a sealed glass jar with water (they've only been been in there
about a week), and it's already been so active that the lid of the jar is pressurized outward. Is this something to worry about? Do I need to let off
the pressure, or will it settle down? |
Go ahead and take the lid off for a moment. Mine never did that, but then I typically make iron gall ink during the winter months so it's probably a
lot cooler when I do it.
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Ok thanks, I'll do that. Hopefully I won't get nasty warm gall tea all over me when I do it!
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Watch them ink beasties!
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
New Research on Iron Gall Ink
I found an interesting 2 1/2 hour video on YouTube by the Library of Congress: New Research on Iron Gall Ink. It goes into the chemistry of iron gall ink. Pretty interesting.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Last year I took a few of the unblemished green black walnuts with husks that had recently dropped off a tree and put them as is on a shelf in my
house. After a few weeks or so the husks shrank/dried into a pleasing crenated hard shape. It's an ornate pattern but there isn't a single photo of
one "developed" to this degree that I could find on Google. I'm going to dry some more now that they are falling off the tree again. They look nothing
like the ugly black husks that appear bruised and decayed as when left on the ground. And while they aren't as exotic as dried poppy capsules or lotus
pods, they are curious in their own way when you hold a few in your hand. The crinkled surfaces are eye-catching, maybe good enough for photo of some
sort.
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
That is interesting, Fiberdrunk! I'm very surprised that they are still uncertain of the actual structure of the molecules! I don't have time yet to
watch the whole thing, but the discussion of the chemical structure begins at 22:40.
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Ink is Finished!
I made the ink! Here is a photo of the ink used in different fountain pens and a glass dip pen.

Only the Hero 5028 felt dry to write with.
I did substitute an equal amount of salicylic acid for the carbolic acid. I also halved the water for fountain pen use (the original recipe was for
dip pens). I added some glycerol as a flow moderator.
By the way, the ink goes down blue but oxidizes to black. It's very waterproof.
Here's how the ink compares with other commercial and homemade iron gall ink recipes:

[Edited on 15-7-2014 by fiberdrunk]
[Edited on 15-7-2014 by fiberdrunk]
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
I wanted to test out how this ink shades. I wrote these samples on Strathmore 100% Cotton using a 3/16" Coit calligraphy dip pen. The ink is slower
to oxidize to black on 100% cotton paper, so this gives you a chance to see how blue it is before it finishes transforming:
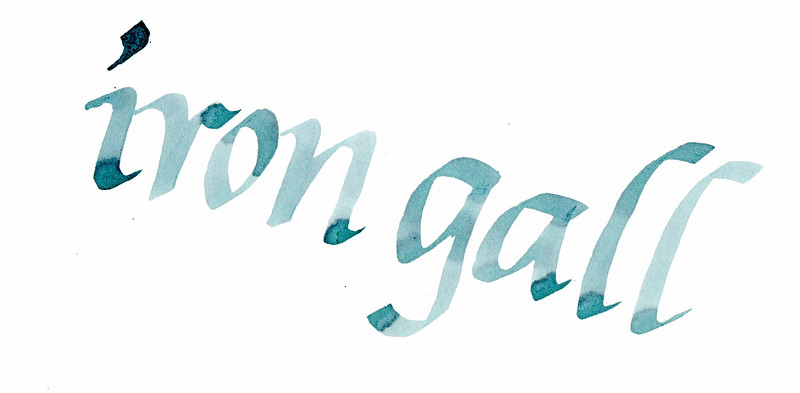
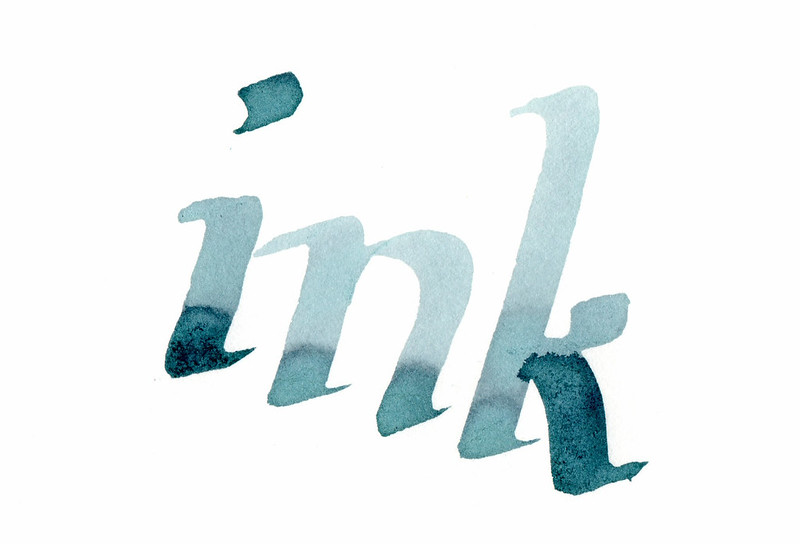
[Edited on 19-7-2014 by fiberdrunk]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Brilliant !
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Thanks!
Here's another sample, this time with fountain pens and a Spencerian flex nib. I don't see much shading with the fountain pens. Again, this is on
100% cotton paper, which is slower to change to black. It gives you a chance to see the blue:

|
|
|
Fenir
Hazard to Self
 
Posts: 68
Registered: 7-5-2013
Member Is Offline
Mood: No Mood
|
|
How well does the ink adhere to the paper? Could you smear the dry ink by rubbing your hand against it?
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fenir  | | How well does the ink adhere to the paper? Could you smear the dry ink by rubbing your hand against it? |
No, it doesn't smear at all. I just tried to rub a pencil eraser over it, too, not just my fingers, and it didn't budge either way. It's very
permanent.
[Edited on 20-7-2014 by fiberdrunk]
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Very pretty pictures, Fiberdrunk!
Is the blue due to the China blue aniline dye? Is it there so you can see the ink before the oxidation starts?
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Artemus Gordon  | Very pretty pictures, Fiberdrunk!
Is the blue due to the China blue aniline dye? Is it there so you can see the ink before the oxidation starts?
|
Thanks! Yes, the blue dye is only there to help you see it in the beginning. On sugarcane papers, the ink rapidly turns to black, almost instantly.
On 100% cotton, it takes about a day or so. I guess you could make the ink without the dye (it's a lot cheaper that way) but you'd be putting down a
rather pale color at first which some may not like. But you ultimately end up with black anyway. I've done it this way, without dye, for the past
few years, with my plant-based iron gall inks. This was actually the first time I've ever used a dye. I had read that dyes compromise the ink in the
long run, causing the writing to brown faster than it would. But I decided it'd be fun to splurge this time and try the dye.
|
|
|
fiberdrunk
Harmless

Posts: 24
Registered: 2-7-2014
Member Is Offline
Mood: No Mood
|
|
Here are the same samples 24 hours later:
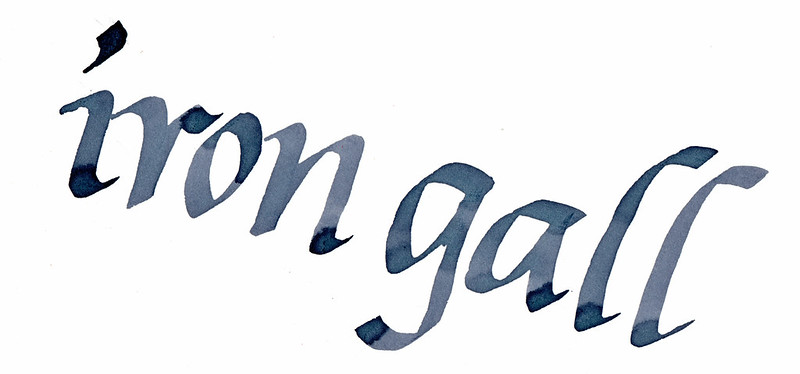
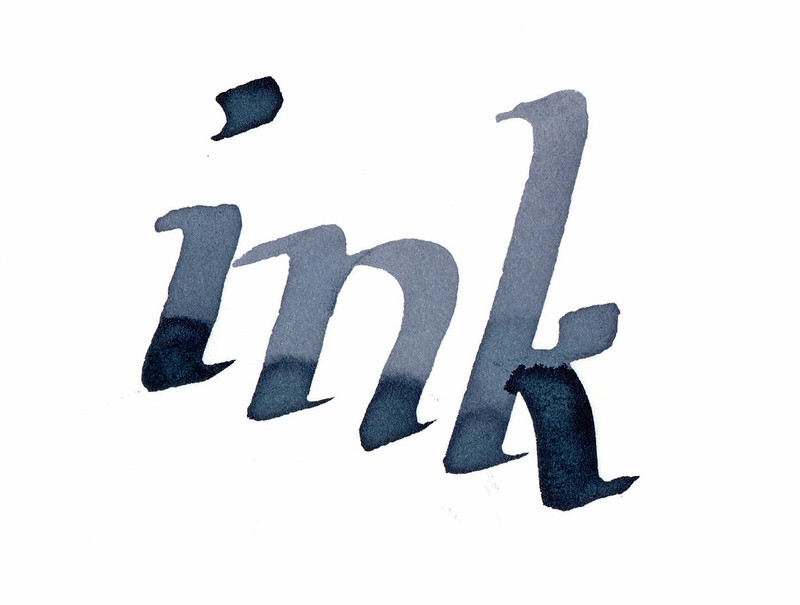
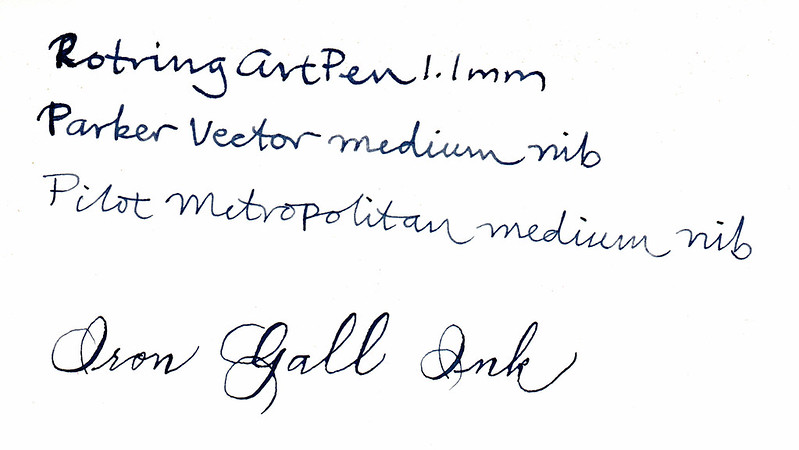
|
|
|
| Pages:
1
2
3 |