| Pages:
1
2
3
4
..
13 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Trinitrotoluene,
TNT is soluble in conc H2SO4 and HNO3; the yield of the reaction is very high from DNT to TNT you should at least get 25g of TNT (and max 27)!
Of course you have to put all the batch in iced water to allow the big % of TNT dissolved in the acid mix and then neutralise the solution with
Na2CO3! You will have much more than 6g then!
If you have trowed away your acid mix, you have wasted at least 19g TNT!!!
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
ive tryed far too many times to make TNT and ended up unsucessful, i have tried HNO3/H2SO4 with heating, KNO3/H2SO4 with heating too, and before that
a few that were cold and stuff, very annoying! it just doesnt seem to want to strp up, i hsould try again. what i should do is make oleum and use that
for the H2SO4 and try to make the HNO3 real good
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
I think I am going to attempt to make TNT. I was thinking that I could pass H2SO4 over anhydrous MgSO4 to produce SO3, which could then be dissolved
in another batch of H2SO4 to produce oleum. The setup would be something like this:
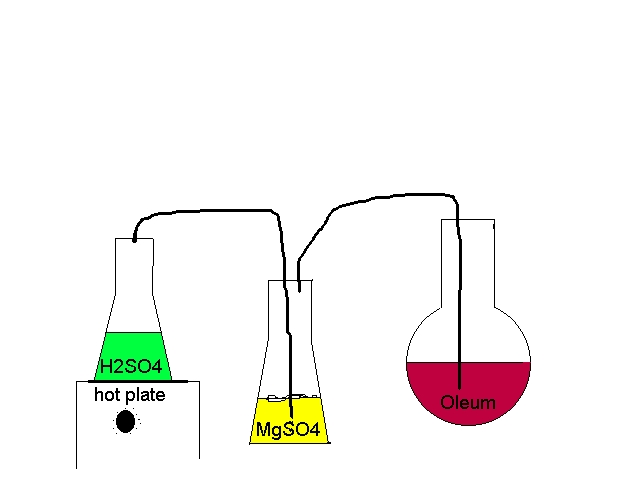
Vir sapit qui pauca loquitur.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Oleum is unneccessary; you can use fuming nitric acid instead ( not as a substitute of course, but along with ordinary H2SO4 ). The reason oleum is
used is that the product from the dinitration along with the waste acids and water from MNT + HNO3 --> DNT + H2O , is used without separation.
So if you just leave the solution of DNT in H2SO4 and HNO3 in the cold overnight most of the DNT precipitates, the acid is decanted ( easy as the DNT
crystals are rather large ) and used for mononitration. The crystals can now be purified and dried, after which they can be dissolved in H2SO4 and
fuming nitric is added slowly.
I suggest using 3.5 mol HNO3 per mol DNT, as the waste acid can then be decanted in the same way as from the DNT and used for MNT --> DNT.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Not exactly TNT preparation, but I didn't find a better place to post this...
TNDB - trinitrobenzodiazonium nitrate. A diazo group instead of the methyl, and a positive charge as a consequence. The negative ion is a nitrate ion.
To prepare this, oxidize TNT to TNBA (trinitrobenzoic acid), neutralize with ammonia, then heat to from an amide. The amide is then Hoffman-degraded,
the resulting trinitroaniline is diazotized and that is then made into a nitrate salt. This has a better OB than TNT and should be more powerful.
|
|
|
tokat
Hazard to Self
 
Posts: 64
Registered: 1-6-2004
Location: 6feet south
Member Is Offline
Mood: No Mood
|
|
mix tublene with nitric acid, if the nitric acid is not 99% then mix it with sulfric acid, what are you lot talking about, that the tub mixes with
three nitro ions, then say it, stop wasting space with crap.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
If I understand that post you think that simply mixing nitric acid and toluene will give you TNT. You are mistaking this with RDX synthesis: nitration
requires the nitronium ion (NO<sub>2</sub><sup>+</sup> ,
which doesn’t exist in straight nitric acid but does in a nitric-sulfuric mix. ,
which doesn’t exist in straight nitric acid but does in a nitric-sulfuric mix.
|
|
|
markgollum
Hazard to Self
 
Posts: 53
Registered: 21-2-2004
Member Is Offline
Mood: No Mood
|
|
You are partially correct neutrino,
The nitronium ion is present in nitric acid, even without sulfuric acid. Just at a much lesser concentration. The nitronium ion is present even to a
lesser extent, in mixtures of nitric acid and acetic acid which are used in the laboratory to nitrate highly activated aromatics to the mono-nitro
stage.( IIRC you can nitrate aniline to mononitro aniline with a mix of glacial acetic acid and nitric acid.)
Also, I am fairly sure that you could make TNT just from nitric acid and toluene, provided that the acid was close to anhydrous (95%+) and that you
used a massive excess of it ( probably 10x at least).
It is understood that the reaction would have to be heated and left to react for a much longer amount of time.
[Edited on 8-11-2004 by markgollum]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The nitronium NO2+ ion would exist in appreciable amounts only in solution in HNO3 which contains additional N2O5 (which is nitronium nitrate), or in
mixtures of HNO3 with concentrated acids which are stronger than HNO3, e.g. H2SO4, HClO4, HPF6.
|
|
|
mark
Harmless

Posts: 25
Registered: 23-10-2004
Member Is Offline
Mood: No Mood
|
|
http://img36.photobucket.com/albums/v109/markthegreat/TNT.jp...
This is taking from the school chem book. Industrail process of Tnt. Perhaps some one is rich enough to make a scaled down version. Now wouldn't
that be cool!
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
I will also post some other preparations of TNT derivatives. I will put all but this one in one file and attach it to a post I will make later. But I
will post this one now.
Triperchloroaminobenzotridiazonium triazide. Souns scary, eh?  Well, basically
it's a benzene ring with three perchloratoamino (a perchlorate group attached to a nitrogen atom with a hydrogen attached to it also, the
nitrogen is attached to the benzene ring) groups and three diazonium ion groups attached. The negative charge is compensated by three azide ions. Well, basically
it's a benzene ring with three perchloratoamino (a perchlorate group attached to a nitrogen atom with a hydrogen attached to it also, the
nitrogen is attached to the benzene ring) groups and three diazonium ion groups attached. The negative charge is compensated by three azide ions.
The preparation is as follows. TNT is oxidized to trinitrobenzoic acid, this is decarboxylated by boiling with alkali, the resulting trinitrobenzene
is reduced in neutral solution (i.e., with zinc and ammonium chloride) to trihydroxylaminobenzene. That is reacted with perchloric acid to give
triperchloroaminobenzene. That is reacted with chloramine to give triaminotriperchloroaminobenzene. This is diazotized with nitrous acid, sulfuric
acid as well to give you triperchloroaminobenzotridiazonium sulfate. That is reacted with calcium azide, which precipitates CaSO4 and leaves you with
triperchloroaminobenzotridiazonium triazide (TPABDA). This explosive has perfect oxygen balance, I expect it to be a crystalline solid, be quite
sensitive and probably more powerful than HMX.
|
|
|
lefty
Harmless

Posts: 9
Registered: 9-11-2004
Member Is Offline
Mood: Blevy
|
|
Something that's been in the back of my mind ever since College organic chem was the conversion of Picric acid to Trinitrotoluene. The
molecules are about the same, with the exception of a hydroxy group in the 1 position in picric acid and a methyl group in the same place on TNT.
If one could only find a way to crack off the OH (thereby forming trinitrobenzene) and replace it with CH3. Massive oversimplification in terms, of
course. I had asked my profs about converting phenol to toluene back when I was in organic chem, and they said that there was too strong of an
affinity for the OH group, ergo...no dice.
It seems like there should be a more efficient way to synthesize the Trinitro though with out multiple nitrations.
Just wishful thinking
[Edited on 24-11-2004 by lefty]
Better to remain silent and be thought a fool than to speak up and remove all doubt.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
You arn't after a more efficient way, you are after an easier one. 3 stage nitration is very efficient.
PCl5 (I think its this and not PCl3) will turn picric acid into picryl chloride. Reacting this with a methyl magnesium should then get you TNT unless
the nitro groups interfere. But even if the method works, and even ignoring the waste of fairly exotic reagents, does it really make sense to make a
fairly safe explosive via a less safe one?
|
|
|
lefty
Harmless

Posts: 9
Registered: 9-11-2004
Member Is Offline
Mood: Blevy
|
|
No... I agree, it's not exactly wise to use a less safe precursor if you don't have to.
Better to remain silent and be thought a fool than to speak up and remove all doubt.
|
|
|
redneck
Harmless

Posts: 12
Registered: 26-9-2004
Member Is Offline
Mood: No Mood
|
|
enter
Megalomanias recipe:
--------------------------------------
EDIT BY VULTURE:
Thank you for supplying us with something we can lookup ourselves easily and which almost everyone knows.
If you haven't got anything relevant to contribute, please STFU.
Thank you.
-------------------------------------------------
[Edited on 24-11-2004 by vulture]
|
|
|
tokat
Hazard to Self
 
Posts: 64
Registered: 1-6-2004
Location: 6feet south
Member Is Offline
Mood: No Mood
|
|
H2SO4+HNO3.H2O(less than 99% + NO) == NH4+ 02+ NO3
HNO3 = NO + NO2+ H
Any updates.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
tokat, what are you going on about?
I suspect you are a gifted scrabble player and that this is over shadowing your chemistry ability.
|
|
|
tokat
Hazard to Self
 
Posts: 64
Registered: 1-6-2004
Location: 6feet south
Member Is Offline
Mood: No Mood
|
|
Think some, then if you still say that is strange, i will have to agreee with you.
Any way i had a reaction that showed the fom, but everone execpt one say i'am wrong, so i guess i am wrong, sorry for wasting the board space.
AAAA, psh, know your you person before hand, CAN ANY ONE ANSWER THE QUESTION??
[Edited on 1-12-2004 by tokat]
|
|
|
CD-ROM-LAUFWERK
Harmless

Posts: 30
Registered: 23-4-2005
Member Is Offline
Mood: No Mood
|
|
the bigest problem i see is the seperation from TNT and DNT
the most preperations of TNT i know doesnt work (like that ones whit HNO3 conc. under 90%)
i also used H2SO4 whit free SO3 and 100% HNO3 but ther wasnt any real TNT formed after some hours of heating
(more than only 2h or something)
the mp. is around 50°C and the stuff can detonat whit a large initiation
can someone make a good instruktion whitin temperaturs, heating times, and so on??
a good speration from DNT and TNT is also needed
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Consult Chemistry and technology of explosives volume 1 (Urbanski) on the FTP, or edonkey and in several languages.
It covers the nitration, destruction of unstable isomers and purification by recrystalisation from a melt.
I'm not a fan of TNT, I dont see the point in making something toxic and difficult to use just because of good press in cartoons.
|
|
|
ChemPhile
Harmless

Posts: 9
Registered: 1-11-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Praetorian
Have anybody knowledge of synthesis of TNT by low concetrated acids and by catalysis of the activated copper? |
I know sth about the mononitration of methoxybenzene by Fe(III) nitrate in the acetic acid. heating is prefered! actually, almost all metal nitrate
occur the same reaction
[Edited on 26-4-2005 by ChemPhile]
|
|
|
magnum
Harmless

Posts: 9
Registered: 26-12-2002
Location: DE
Member Is Offline
Mood: No Mood
|
|
there is a patent (us2435314) wich shows an other method for tnt preparation. in this process no sulfuric acidis needed and the nitric acid has not to
be concentrated. does anyone know this procedure or has anyone tryed it?
|
|
|
CD-ROM-LAUFWERK
Harmless

Posts: 30
Registered: 23-4-2005
Member Is Offline
Mood: No Mood
|
|
i seperate the TNT now from the DNT by melting the stuff and coolig slowly down, the TNT crystalize out and and u can remove the liquid DNT (the wory
is that the TNT is a bit souble in DNT)
but after 1 week of sometime heat a mixtur of fluming H2SO4/100% HNO3 ther are only around 10% nitrated DNT....
how to get a high-yield (and a fast one) nitration??
|
|
|
Taaie-Neuskoek
Hazard to Others
  
Posts: 222
Registered: 14-5-2004
Location: Zermany
Member Is Offline
Mood: Botanical!
|
|
| Quote: |
there is a patent (us2435314) wich shows an other method for tnt preparation. in this process no sulfuric acidis needed and the nitric acid has not to
be concentrated. does anyone know this procedure or has anyone tryed it?
|
With a google search I can found this, posted on E&W a few years ago, by 'MACE':
| Quote: |
there is an other method of TNT preperation described in the patent US2435314. if this really works this would be very interesting because there is no
need of concentrated nitric and also sulfuric isn't needed at all. the process is something like this: you have to put the nitric (concentration
dosn't matter, just calculate the right ammount for the aviable concentration) , the toluene and a solvent (light gasoline, n-hexane?) in a
reflux aparature equipped with a watertrap and boil it until the calculated ammount of water has collected in the watertrap. the temperature will
maintain it self at ~70°C because of the boilingpoint of the solvent. this will also prevent a runaway because if the temperature rises more solvent
evapoates and this cools the reaction. i don't have tryed out this method until now but i'm on to obtain the equipment needed.did anyone
tryed out this method befor?
|
This looks like the 'one pot TNT synthesis' NBK resenty posted, which uses a solvent which should boil with toluene at around 70°C, a
Dean-stark trap can be used to trap the water. I haven't tried anything of this myself, but it seems rather promising...
EDIT: Thanks, Joeychemist!
[Edited on 4-5-2005 by Taaie-Neuskoek]
Never argue with idiots, they drag you down their level and beat you with experience.
|
|
|
magnum
Harmless

Posts: 9
Registered: 26-12-2002
Location: DE
Member Is Offline
Mood: No Mood
|
|
jea this was me in the e&w who has written this post but it seems that no one was interested in this procedure. i thought of talking about this
patent again because i now have the equipment and chemicals to give this a try. it's cooking otside while i write this. i use hexane as solvent
and a nitric acid with 53% concentration.now there realy warter collects in the wartertrap and the liquide in the flask has turned yellow.
i don't know the 'one pot TNT synthesis' from NBK do you have a link to this?
|
|
|
| Pages:
1
2
3
4
..
13 |