| Pages:
1
2
3
4
5 |
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
no I do not own a neutron source , but I was considering building an alpha linear accelerator with a thin Be sheet I have..
the sensitivity ? let me put it this way, the sodium iodide crystals let me detect K40 in a pound of potassium hydroxide
the Cadmium Telluride diode lets me pick up the x rays from a cathode ray tube instantly, and the High purity Germanium detector I just order (if it
works) would let me see through a complicated spectrum of multiple isotopes.
the sensitivity is not the problem. with enough time I could pick out a few thousand atoms (or less) given they are all radioactive.
here is an (old) source of Cobalt 60 with the NaI(Tl) crystal
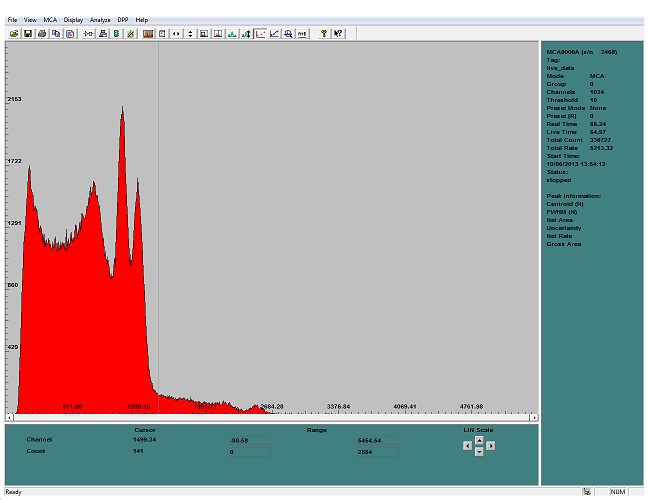
the two peaks are clearly separated and visible
here is ba133 with the CdTe detector
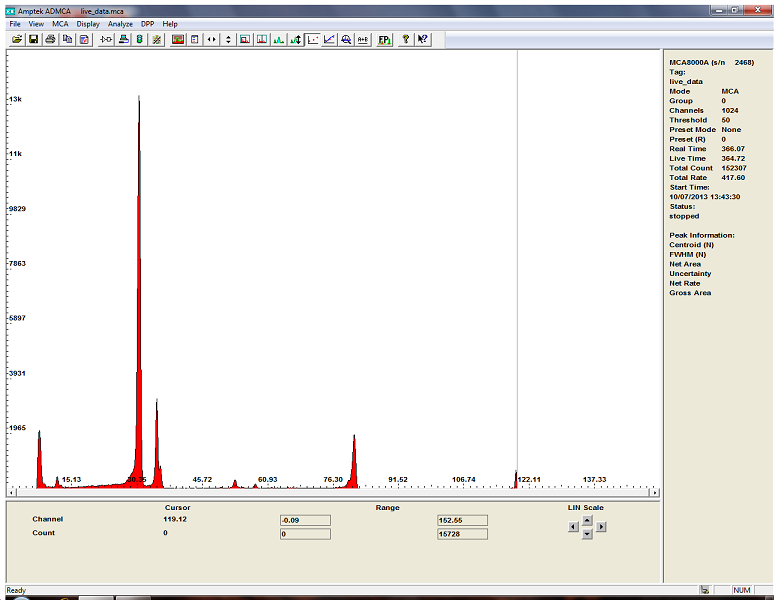
and this is Americium 241...
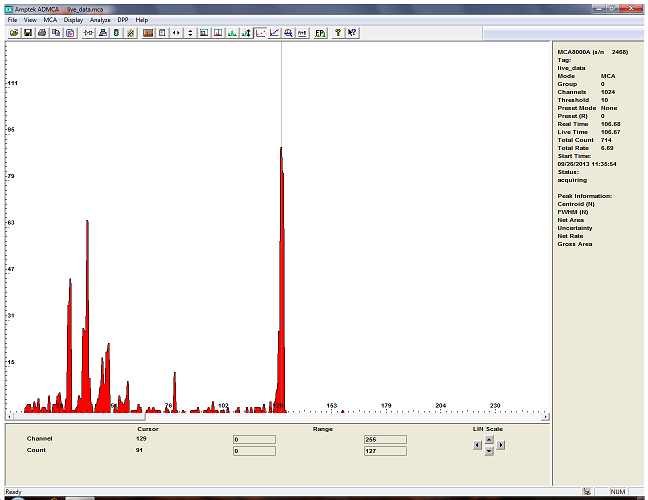
I will post more when I get the HPGe detector and some liquid nitrogen
let me be clear, I cannot detect molecules or stable isotopes and a neutron activation would depend on the cross section of the element you are trying
to activate .
also some isotope generated by neutron activation have a really short half life and cannot be shipped for analysis nothing would be left to detect!
only long lived isotopes (T/2>about 10 hours) can be successfully detected.
[Edited on 17-12-2013 by neptunium]
|
|
|
bfesser
|
Threads Merged
16-12-2013 at 17:32 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Yes. Done.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I've got an unknown metal alloy that has stumped me on the wet analysis. I would love to have you take a look at a sample.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
sure! if I can activate it that should be no problem! u2u me I`ll tell you where to send it...
please understand that I travel a lot for work and might not be able to perform the analysis for some times.
hope your not in a hurry!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I think this is really cool but I don't think you'll get the exposure you deserve due to the thread title. Change it to "Anyone interested in
radioisotope analysis" or the like and I'm sure you'll improve your audience greatly. It might even be more suitable to move this to "Chemistry in
General", as topics in Misc quite often don't get the attention they deserve.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
This isn't a Chemistry topic. I agree with changing the title, though. <strong>neptunium</strong>, just let me know.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes I was trying to change it to radio isotopes identification, but couldn't figure out how to do it!
and it should have been in technochemistry maybe?
sorry I `ll be more careful next time .
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I'm concerned by the hand image and the lack of shielding around the bare X-ray tube. Is that a film exposure or a fluoroscope? Have you calculated
exposure?
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
I am not using it as a XRF I was just testing the detector response...it would be too dangerous to run it naked like the picture! yes I have
calculated the exposure...that's why I stopped!
Beside the tube heats up a lot and wouldn't function very long anyway..
So NO X ray spectroscopy ! sorry
[Edited on 17-12-2013 by neptunium]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
....gonna do some neutron activation soon though! 
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i got this 0.5mCi of Po210 on ebay so i made a poor man`s neutron source to see if i could activate this sample of mystery metal sent by hyfalco and
this is what i got...
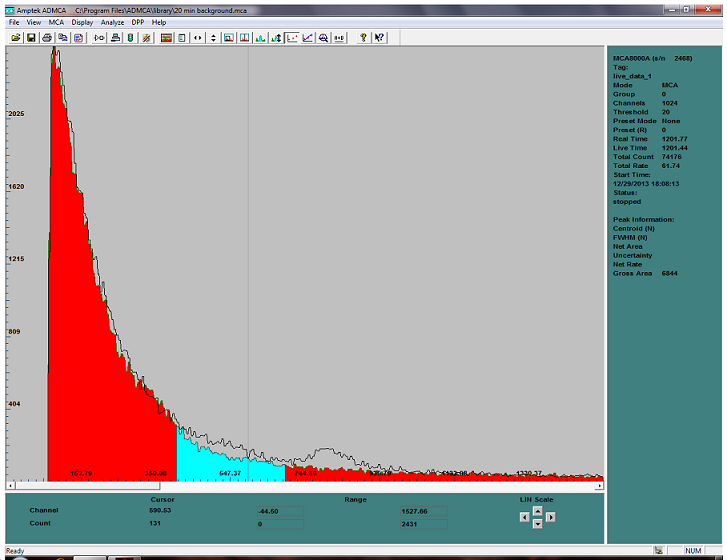
this is only about 30 minutes of exposure in a neutron oven... the 809Kev peak of the Po210 is clearly visible ,
however the area highlighted before that in the ROI (Region Of Interest) is particulary interesting...
arround 400 to 600 Kev is where the radio isotope of the mystery metal lay...
i am going to let it sit over night and see if anything else shows up on the gamma spectrum...
note
the red area is a background radiation without anything in front of the detector, the spectrum overlay on the background lets us appreciate the
difference between the two spectrums and allows us to see minute amount of radiations..
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i ran the analysis again with the x ray detector and i think the sample must contain Ta (tantalum)
the stable isotope is Ta181 and after neutron activation we should expect to find Ta182.
although the activity is barely above the background radiations, most of the expected gamma and X rays are present !
this is not to say that ta is the only element in the sample but rather the most neutron sensitive (higher cross section).
here is the x ray spectrum compare with the background.
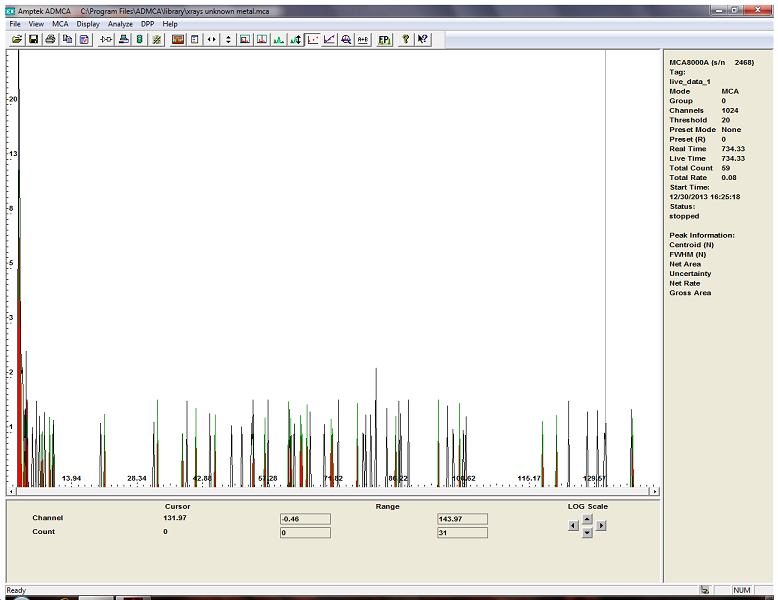
i know it might not look like much but its pretty hard to mess up the calibration on this detector so i remain confident that Ta is present.
i dont know where the sample came from but Ta is often use in alloys because of its chemical stability 9corrosion resistant ) so it doesnt really
suprise me .
what does suprise me is that i was able to detect it ! this is a very powerful and non destructive method of analysis and i find it mind blowing!
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Very cool. If you want, I can supply you with an FTP account to upload full resolution images (>800px). It would make it much easier for us to
see what's going on in these spectra. Just U2U me if you're interested.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes the resolution is sort of crapy but we can kinda tell the energy scale at wich peaks occurs ...
again this is a very powerful non destrutive analytical way of determining elements presence. every chemist on here should be all over this!!!
[Edited on 30-12-2013 by neptunium]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
neptunium (or anyone interested), I found these <a
href="http://www.ebay.com/itm/Hamamatsu-X-Ray-Phosphor-Screen-With-Fiber-Optic-New-/131069478644" target="_blank">phosphor screens</a>
<img src="../scipics/_ext.png" /> while searching for PIN photodiodes to use in <a
href="viewthread.php?tid=25882&page=3#pid313842">γ-ray detection</a>, and thought you might find them useful for amateur X-ray
imaging applications. The seller claims they're also somewhat sensitive to β. [edit] Check out their other mad science type items, I see
some nice stuff.
[Edited on 3.1.14 by bfesser]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
interesting indeed... i used a similar screen for the x rays (previous pictures)
some of them glow green others blue ... but the amount of radiation needed for the glow to be visible is colosal.
alpha particles can be viewed with a weak source (few micro curies) on a spintariscope if you let your eyes adapt to the darkness..
i would love to know what type of phosphor he uses, although no spectroscopy can be done with a scintillator this thin, for 30 bucks i might give it a
shot!
[Edited on 4-1-2014 by neptunium]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
got a new bigger detector !!!!! this a a harshaw "well type" so 360 degrees detection for more accurate sample analysis !

I did a quick 15 minutes background ( black line ) followed by a 15 minutes of 2 lbs potassium hydroxide ...
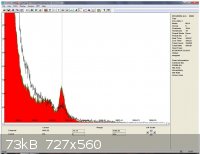
don't let the size and resolution of the picture fool you this is natural radioactivity very visible! no doubt about the 1460Kev peak !
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Very nice. I'm not familiar with what a Harshaw "well type" detector is. Does this put a size limitation on the sample dimensions (does the sample
need to fit inside a well in the detector)?
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
you got it ! It may sound like a restriction but you gain on activity measurement (detect faster ) since the sample is "inside " the scintillation
material.
However it works just as well with a bigger sample like the 2lbs jar of KOH just sitting on top of the detector. Harshaw is just the brand name like
Bicron or canberra
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I see. I did look up the Harshaw name, and found a site for old employees of the company, but not much on the detectors. What are the well
dimensions of your new detector?
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
the number on the detector are 12SW12-w4 the hole is about 2 in deep and 3/4 in wide
these little glass bottle are a perfect fit.

|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
check out this beautifull well defined Co60! the materialisation peak at 511Kev is clearly visible!
<a href="http://www.scimad.org/users/neptunium/Co60.jpg"><img src="http://www.scimad.org/users/neptunium/Co60.jpg" width="200"></a>
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: hosted
image(s) at scimad.org/scipics2/]
[Edited on 2.2.14 by bfesser]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
been playing with this data analysis software fitzpeak to look at my spectrum and it seems like a powerful tool!
here is the report after calibration andanalysis
Attachment: Main Report.txt (3kB)
This file has been downloaded 1337 times
and here is the detail spectrum
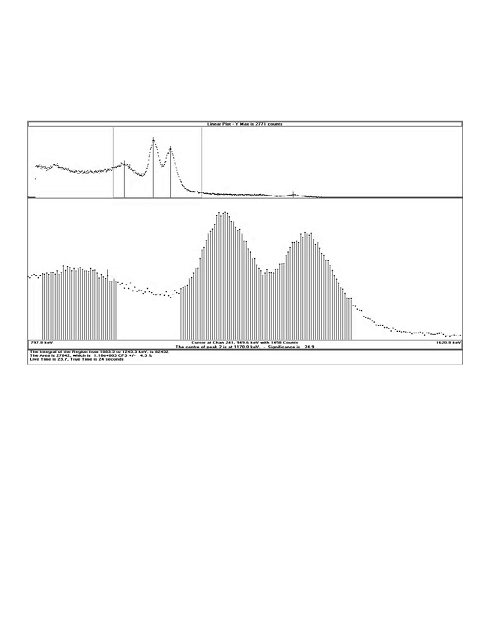
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
for the one or two interested i am boiling off snow (yes it snowed again) because i read snow is a good filter
for atmospheric dust including radio active nuclide!
so when i have enough boilled off i 'll run an analysis to see what kind of radio pollutant i could find!...
stay tuned for updates! soon....
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Interesting. The graphs you're posting are difficult if not impossible to interpret, due to the images having been resized. Please look into a
better way of posting images. (I've explained several methods in previous topics, and think I even sent you a U2U with FTP account information.) At
the very least, email copies of the full resolution images to me, and I can upload them and edit them into your posts, on your behalf.
|
|
|
| Pages:
1
2
3
4
5 |