| Pages:
1
2
3
4
..
6 |
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
On the subject of immortality
Terbium will not die.
My mentor for the fair took the terbium to his house and tried to break it. He used a saw, a knife, and all sorts of other tools. The only thing that
worked was a chisel. We now have small pieces, as well as a large chunk of terbium that made it.
I attempted the synthesis yesterday. 0.48 grams of terbium was added to 1.14 grams of iodine dissolved in 20 mL of methanol. Not all of the iodine was
added at once because I wanted to monitor the reaction to see any signs. I added about 20 mg initially, but that was enough to turn the solution a
very dark brownish-purple. I added in all of the terbium, but it was very difficult to see with the amount of iodine in solution. A few bubbles were
escaping from solution. In order to accelerate the reaction, I added the rest of the iodine. The reaction did not appear to speed up at all.
In a test tube, I decided to perform a test with small flakes of iodine and terbium (exact amounts were unknown) and distilled water (about 2 mL) was
added. The solution became yellowish brown and bubbling began. Not all of the iodine dissolved. The two solutions were left to sit for the next day.
The next day, no change appeared in the methanol beaker. The solution remained extremely dark, and terbium was visible at the base of the flask.
Something completely different happened in the test tube with water. The water had mostly evaporated, leaving behind a froth. On the addition of
water, it became apparent that all of the terbium had reacted with the iodine solution. No oxide powders appeared to be in the solution. I added in
more terbium and water to react with the iodine. After an hour or so, the terbium flakes were bubbling, and a very fine white powder appeared to be
suspended in the solution. I forgot to check for fluorescence (terbium oxide exhibits green fluorescence), so I can't be sure if that was the oxide or
something else.
I won't be experimenting next week, but I'll update soon.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Quote: Originally posted by Brain&Force  | | Something completely different happened in the test tube with water. The water had mostly evaporated, leaving behind a froth. On the addition of
water, it became apparent that all of the terbium had reacted with the iodine solution. No oxide powders appeared to be in the solution.
|
So what did you get? A clear solution dark coloured due to a KI-I like iodine-complex?
Quote: Originally posted by Brain&Force  | | I added in more terbium and water to react with the iodine. After an hour or so, the terbium flakes were bubbling, and a very fine white powder
appeared to be suspended in the solution. |
This sounds as if no free iodine was present anymore. Is this true? If this product doesn't dissolve then you should have something different than in
the first case described above!
Quote: Originally posted by Brain&Force  | | I forgot to check for fluorescence (terbium oxide exhibits green fluorescence), so I can't be sure if that was the oxide or something else.
|
This isn't a good way to check your product. TbCl3 also shows green fluorescence. TbI3 should dissolve in water extremely well
but the oxide does not. So this is the easiest way to check your product. But: some rare earth halides form oxy or hydroxy halides when heated in
water. This is what you may have produced in the second case (white precipitate)
Here is a paper with solubility data for the rare earth halides (except fluorides). I don't understand Fig. 1 there, but this may be the basis to
calculate the solubility of TbI3. Does anyone understand this figure and can give an example for the calculation of the solubility of one
salt?
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I wonder if terbium would become brittle, harder, or unaffected if placed in liquid nitrogen?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ Pok:
Great find [that paper]! I believe, gathered from the text, that 'x<sub>LnX3</sub>' stands for 'mole fraction of
LnX<sub>3</sub> in the solution'. A very thermodynamics type of solubility measuring unit. It can be easily converted to the more
traditional 'g / 100 solvent'.
@ B&F:
In either case, did you notice any heat being generated?
"No oxide powders appeared to be in the solution." Does this mean the solution was clear?
If the bubbling wasn't due to boiling then it's likely to be hydrogen (you can check that of course) which would indicate you're getting the water to
oxidise the Tb. Possibly to the hydroxide Tb(OH)<sub>3</sub>, which may then react with the iodine to form iodide + iodate:
3 I<sub>2</sub> + 3 OH<sup>-</sup> === > 5 I<sup>-</sup> + IO<sub>3</sub><sup>-</sup> +
3 H<sup>+</sup>
... according to the classical preparation of iodide from iodine and a strong alkali. As a hypothesis it would at least explain why you get bubbles
AND the iodine reacts away.
When it comes to the direct union of Tb and I<sub>2</sub> in a solvent and at RT, this may not necessarily start without heat: 'activation
energy' may be needed.
@ Morgan: it will become more brittle, of course. But a practical way of size reducing this ain't, at the home level.
[Edited on 23-11-2013 by blogfast25]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | | I believe, gathered from the text, that 'x<sub>LnX3</sub>' stands for 'mole fraction of LnX<sub>3</sub> in the solution'. A
very thermodynamics type of solubility measuring unit. It can be easily converted to the more traditional 'g / 100 solvent'. |
And how? Please tell me if the following calculation is correct:
Example: NdCl3
Solublity of NdCl3 = 967 g/1000 ml at 13 °C.
967 g NdCl3 = 3,85 moles
1000 ml water = 55.5 moles
Sum of moles = 59.35
moles NdCl3 / total moles = 3,85 / 59.35 = 0.0649 = the "x" ?
According to Fig. 1 (see below) "x" would be ca. 0.063 at 25 °C.
This sounds correct, right?
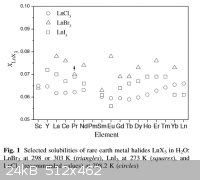
Solubilities of rare earth halides
[Edited on 23-11-2013 by Pok]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ok. Suppose a binary system of A and water, with x<sub>A</sub> the mole fraction of A. Call n<sub>A</sub> the amount of A in
moles and n<sub>H2O</sub> the amount H2O in moles . Then:
x<sub>A</sub> = n<sub>A</sub> / (n<sub>A</sub> + n<sub>H2O</sub>
and:
x<sub>H2O</sub> = n<sub>H2O</sub> / (n<sub>A</sub> + n<sub>H2O</sub>
It follows that x<sub>A</sub> / x<sub>H2O</sub> = n<sub>A</sub> / n<sub>H2O</sub>
Also: mass balance: x<sub>A</sub> + x<sub>H2O</sub> = 1
Assume you have 100 moles of mixture (or any number, for that matter), that contains 100 . x<sub>A</sub> moles of A and 100 . (1 -
x<sub>A</sub> moles of water. moles of water.
Multiply both numbers of moles by molar mass to obtain weight in g (say A g and H2O g). Divide A g by H2O g and multiply by 100 to obtain gram of A
per 100 g of water.
The hydrates of TbI<sub>3</sub> could be a serious hurdle for the OP's project: dehydrating them w/o hydrolysis is going to be nigh
impossible I feel. It's hard enough for the chlorides, never mind the more fragile iodides.
[Edited on 23-11-2013 by blogfast25]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Ok. This sounds as if my last post is right. Correct me if this is wrong.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No, you are right. It's easier to do it from first principles though, IMO.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Excuse my previous post, utter nonsense, completely misread that you got the reaction with the water version, thought it was all methanol. 
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I need to clarify something else. The white powder was like suspended dust, very sparse and hard to see. I can't be sure if that was
Tb(OH)3 or something else, which is why I wanted to use the UV light to check. If it was Tb(OH)3 the stuff would have glown
green.
| Quote: |
So what did you get? A clear solution dark coloured due to a KI-I like iodine-complex? |
That's what I'd guess. The solution was dark yellowish brown because of iodine, but there was still some undissolved iodine at the bottom.
| Quote: |
This sounds as if no free iodine was present anymore. Is this true? |
There was still dissolved and undissolved iodine in the test tube. Sorry, I should have clarified that also.
| Quote: |
Here is a paper with solubility data... |
Thanks for the paper; this might be useful for my write-up for the fair.
| Quote: |
In either case, did you notice any heat being generated? |
I didn't feel any heat in the methanol solution; I just saw bubbles being generated. The iodine solution in water had a lot more bubbling activity.
| Quote: |
"No oxide powders appeared to be in the solution." Does this mean the solution was clear? |
Correct.
| Quote: |
If the bubbling wasn't due to boiling then it's likely to be hydrogen (you can check that of course) which would indicate you're getting the water to
oxidise the Tb. Possibly to the hydroxide Tb(OH)3, which may then react with the iodine to form iodide + iodate:
3 I2 + 3 OH- === > 5 I- + IO3- + 3 H+
... according to the classical preparation of iodide from iodine and a strong alkali. As a hypothesis it would at least explain why you get bubbles
AND the iodine reacts away. |
True, but I thought this equilibrium exists between iodine and water:
I2 + H2O ↔ HI + HIO
and that this would acidify the solution so that the Tb would be able to dissolve in the solution rather than precipitate as Tb(OH)3. As
for the formed hypoiodous acid, it would either reduce the Tb or disproportionate to I- and IO3-. If I have iodate,
how do I reduce it to iodide?
| Quote: |
When it comes to the direct union of Tb and I2 in a solvent and at RT, this may not necessarily start without heat: 'activation energy' may
be needed. |
I thought the reaction would be immediate because of terbium's extremely negative reduction potential. But for a supposedly reactive metal, terbium is
quite inert! I'll try direct union (heating Tb and I2 in a test tube on a VERY small scale) and I'll see what happens.
| Quote: |
Excuse my previous post, utter nonsense, completely misread that you got the reaction with the water version, thought it was all methanol.  |
I did get bubbling in the methanol solution, but it wasn't much. I know the methanol is dry (it says absolute methanol on the label). I'd be surprised
if terbium formed a methoxide. I'll leave a piece of terbium in methanol and see what happens.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
If all else fails, perhaps a round about experimental route:
Tb pieces are reacted in/with excess glacial acetic acid to form terbium acetate solution in glacial acetic acid. The rationale here is that terbium
acetate is probably soluble enough in acetic acid, the system is anhydrous and the acetic acid should make quick work of any surface oxides on the Tb
that may otherwise have passivated it in anhydrous conditions.
Then bubble through a slight excess of HI and finally filter hopefully insoluble TbI3 that forms.
Finally evaporate under prolonged vacuum to recover acetic acid free TbI3 and get rid of everything else (acetic acid and HI).
The reason I suggest this route and not using a preprepared HI/acetic acid solution is because I expect TbI3 not to be particularly soluble in glacial
acetic acid.
However, EVEN if soluble, prolonged vacuum will strip off the acetic acid anyhow, it will just take longer.
Just remember to be kind to your vacuum pump and use an inline cold trap! These vapours would be corrosive as hell! Liquid nitrogen is best but even
dry ice should work well enough in this case.
[Edited on 23-11-2013 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
HI is difficult to synth/obtain. Might as well start planning for direct union: Tb + I<sub>2</sub>. Lead I<sub>2</sub>
vapour/Ar mix over size-reduced Tb metal in quartz tube.
[Edited on 24-11-2013 by blogfast25]
@ B&F:
In the synthesis of iodide/iodate from KOH and I2, the iodate is converted to iodide by heating. But there we start from an anhydrous product...
Re. the electropositivity of Tb and activation energy: it doesn't automatically follow that an electropositive element will combine spontaneously with
an electronegative one without increasing temperature. The Tb doesn't spontaneously combine with oxygen, for instance. Clearly the
Tb/I<sub>2</sub> system is different from Draconic students' Zn/I<sub>2</sub> system.
[Edited on 24-11-2013 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Metal halogenations in furnaces are no joke. While the furnace is relatively 'easy' to get a hold of, making the innards for it, typically of fused
quartz, is not.
If HI is cumbersome, what about a simple ion metathesis using sodium iodide added to the terbium acetate/acetic acid solution. NaI is pretty soluble
in many organic solvents. One would need to look up all the solubilities preferably if they are available to design the experiment properly. Here's
one:
From wiki's article on NaI:
Solubility of NaI in various solvents
(g NaI / 100g of solvent at 25°C)
H2O 184
Liquid ammonia 162
Liquid sulfur dioxide 15
Methanol 62.5 - 83.0
Formic acid 61.8
Acetonitrile 24.9
Acetone 28.0
Formamide 57 - 85
Acetamide 32.3
Dimethylformamide 3.7 - 6.4
Dichloromethane 0.009
Solubilities from:
Burgess, J. "Metal Ions in Solution" (Ellis Horwood, New York, 1978) ISBN 0-85312-027-7
Danil de Namor, A.F.; J. Chem. Soc., Faraday Trans. 1, 1989,85, 2705-2712 DOI: 10.1039/F19898502705
I could not find data for the solubility of sodium acetate in acetic acid but surely it exists somewhere.
Still also need to know the solubility of TbI3 and acetates in acetic acid (preferably).
Anyhow, trying to dissolve some terbium in glacial acetic acid should be trivially easy. Then adding a saturated solution of NaI in glacial acetic
acid will either yield a coloured precipitate or not. Simple enough to try, no?
[Edited on 24-11-2013 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ Delta:
I think it gets complicated rather quickly. Your GAA would have to be truly anhydrous for instance. The stuff I've got is 95 +, IIRW.
Direct union of iodine vapour and terbium isn't for the faint of heart either but marginally easier (I think) than what you propose. I guess it
depends: some are much easier than others.
[Edited on 24-11-2013 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yeah ok... the op's got some options then and we've done our job, but I don't see why the average run of the mill GAA wouldn't be just fine in this
case. Is it not ordinarily pretty anhydrous?
Just checked sig-al's GAA's spec sheet, it says water <= 1500ppm, surely that's fine, or is CP grade GAA normally much worse than this? This is
your area of expertise 
Ah ok, saw you edited yours as well  95%... that's not very good, is the 5% all
water? I wouldn't exactly call that 'glacial'? 95%... that's not very good, is the 5% all
water? I wouldn't exactly call that 'glacial'?
[Edited on 24-11-2013 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Actually, I told an inadvertent lie: mine says 99.85 % (AA assay). Assuming the rest is water that would be about 1500 ppm, pretty good.
Re. Sig Al: good luck buying from them. I run a chem business and they still won't well to me. I'm going to appeal just to rattle their cage a bit.
As far as stabs in the dark go, your idea would be worth testing.
[Edited on 24-11-2013 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you have acetic anhydride, then you can make your glacial acetic acid really free of water. I have 250 ml of glacial acetic acid and I added 1 ml
of acetic anhydride to be sure that it is absolutely free of water.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Blogfast
Yeah same specs then, I knew you run a chem business  I also expected the sig. al.
comment lol I'm no fan either for the record! I also expected the sig. al.
comment lol I'm no fan either for the record!
Nice trick woelen!
[Edited on 24-11-2013 by deltaH]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
The GAA idea is a good one, but there's a problem. I'm working in a school, and as far as I know we don't have glacial acetic acid. (Also, acetic
anhydride is prohibited, so I can't dry it.) So I might try direct union as blogfast25 proposed (if the iodine water/terbium mix didn't work.) Now
I'll have to calculate hydration numbers and things (and I can't find a source for the hydration of TbI3, but I have found terbium chloride
hexahydrate and terbium bromide hexahydrate mentioned in the abyss of the interwebs).
I was considering HI, but that stuff is hard to get or make and it raises eyebrows everywhere...
This is getting really complicated really fast.
On a different note, I found this short paper on a terbium-cobalt redox system in aqueous solution. Apparently terbium can be plated out of aqueous solution if cobalt is present.
Quite unusual for such a reactive metal.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
B&F:
The GAA is easy to obtain and cheap. Buy it yourself from eBay.
The degree of hydration and solubility of TbI3 are presented in the paper linked to by ‘pok’, higher up. The REs form octahydrate iodides, so e.g.
TbI<sub>3</sub>.8H<sub>2</sub>O. Fig. 1 tells us that for that salt, at saturation the mole fraction of
TbI<sub>3</sub> in the saturated salt-water system (at 25 C) is about 0.065. You can then convert that to the more traditional g
TbI<sub>3</sub> per 100 g of water, as outlined above.
The real problem with such high hydrates then is to get rid of the crystal water. For an iodide that would probably mean heating in vacuum to reduce
the temperature at which the water leaves the crystal lattice and thus to prevent hydrolysis to a mess of hydroxy iodides. Oxygen needs to be avoided
to avoid oxidation of the iodide to iodine.
Dehydration of the REX3 compounds is generally not easy. Of course your project might not require anhydrous TbI<sub>3</sub>, I don’t
know that.
[Edited on 30-11-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Some success! But...
My whole post (quite a long one) got deleted when I previewed it. I'm not going to bother writing it up again (unless you want me to).
In short, I made some terbium iodide through direct union and heating. The product was confirmed by hydrolyzing a solution of it, precipitating
terbium(III) oxide and some higher oxides. I didn't expect this to happen on hydration.
Enjoy the picture of terbium ions fluorescing green in solution.

I didn't expect this to occur. After watching mrhomescientist's video on making terbium nitrate, I found a comment stating that the salt didn't
fluoresce in solution. Perhaps the anion can make a difference (there was iodide and triiodide in solution.) I'll try terbium chloride and/or the
acetate.
Better pics to come soon.
[edit] Blogfast, thanks for the note on LnI3 hydration. But I cannot access the paper.
[Edited on 5-12-2013 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Fascinating, congratulations on carrying out a direct union. When you feel up to it, I am sure we would love to hear how you did this! Sorry about the
deletion 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | My whole post (quite a long one) got deleted when I previewed it. I'm not going to bother writing it up again (unless you want me to).
|
Can I insist on a short version of a write up? Tip: always save something longish in your favourite word processor before submitting here.
I'm surprised it seems to hydrolyse so easily and am wondering if you're perhaps seeing something else. If there's a next time, try acidifying the
solution a little with acetic acid (I'm assuming the acetate is very soluble, so won't crystallise when you want to try and recrystallize the
TbI<sub>3</sub> . .
TbCl<sub>3</sub> hydrate:
For TbCl<sub>3</sub>, you can dissolve the Tb directly in 37 % HCl.
At RT the mole fraction of TbCl3 in a saturated solution is about 0.06 (acc. that paper). That works out at 94.1 g TbCl3 / 100 g water (the MM of
TbCl3 is 265.28 g/mole).
Dissolving stoichiometric amounts of Tb in HCl 37 % would give you about 142.3 g TbCl3 / 100 g water (assuming no loss of acid or water), so already
above the saturation point. On cooling quite some TbCl3 hydrate should form, because much water will be tied up in the hydrate.
To dissolve the Tb in the acid, add small lumps of the metal to the acid, expect much effervescence and heat. Cool between additions if needed
(probably, at least at first!)
And take pictures! You don't get to see that kind of thing everyday!
[Edited on 5-12-2013 by blogfast25]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | In short, I made some terbium iodide through direct union and heating. The product was confirmed by hydrolyzing a solution of it, precipitating
terbium(III) oxide and some higher oxides. I didn't expect this to happen on hydration.
...
I didn't expect this to occur. After watching mrhomescientist's video on making terbium nitrate, I found a comment stating that the salt didn't
fluoresce in solution. Perhaps the anion can make a difference (there was iodide and triiodide in solution.) I'll try terbium chloride and/or the
acetate. |
Awesome work! I'm glad you saw my video, too. When I made the nitrate, it definitely did not fluoresce while in solution - only the crystals. To make
sure I understand your process (I'd love to see a writeup too): you heated elemental iodine and terbium together to form the triiodide, then added
this to water to precipitate terbium oxides. Are these somewhat soluble? I.e. how did terbium ions get into solution to enable it to glow?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | | To make sure I understand your process (I'd love to see a writeup too): you heated elemental iodine and terbium together to form the triiodide, then
added this to water to precipitate terbium oxides. Are these somewhat soluble? I.e. how did terbium ions get into solution to enable it to glow?
|
The hydrolysis, if that is what really took place, is only partial. So some Tb hydroxychloride/Tb hydroxide may have formed but there will be plenty
solvated Tb<sup>3+</sup> left.
Unless oxygen got to the Tb during the direct union, of course. W/o vacuum/argon that cannot be excluded a priory.
[Edited on 5-12-2013 by blogfast25]
|
|
|
| Pages:
1
2
3
4
..
6 |