| Pages:
1
2
3
4
..
77 |
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Haloform reaction
(Iodoform)

|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
That's a heckuva lot of <a href="http://en.wikipedia.org/wiki/Iodoform" target="_blank">iodoform</a> <img src="../scipics/_wiki.png"
/>! You must have a use in mind; care to share the details?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Sublimated phthalimide in a large test tube, thought it was neat 
***edit*** Looking at it now, it reminds me of a dark animal eye. Weird.

[Edited on 12-10-2013 by Mailinmypocket]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by bfesser  | | That's a heckuva lot of <a href="http://en.wikipedia.org/wiki/Iodoform" target="_blank">iodoform</a> <img src="../scipics/_wiki.png"
/>! You must have a use in mind; care to share the details? |
That is 50gr of Iodoform.
I sell it to my friend (dentist) and he use it as disinfectant
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
We just ran out of deuterated solvents for NMR, so we had to make some. Here is a "few" ampules of mixed deuterochloroform/deuteromethanol with a
little added tetramethylsilane for NMR spectroscopy.

I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
palico
Hazard to Self
 
Posts: 63
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Simply sodium chloride crystal grown on a stone.

|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
The iodoform is really cool looking. It reminds me of lead iodide which I just made. I have so many beautiful compounds and elements. I wish I had a
camera.
|
|
|
I Like Dots
Hazard to Self
 
Posts: 69
Registered: 10-4-2013
Member Is Offline
Mood: frisky
|
|
Some nickel/copper acetate I made


|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Very nice! Careful handling those nickel compounds, though! 
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Just another day at the office...

[Edited on 18-10-2013 by DJF90]

|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I'm salivating over that large-scale setup. Can't quite make out the labels. What'cha got cookin'?
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
aah bfesser, you too?
the one one the right looks like triethylamine
all above information is intellectual property of Pyro.  |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
I *think* that on the left is triethylamine, then sulfur trioxide (something..) complex and dimethyl sulfoxide. Might be wrong but I wanted to take a
guess lol. I wish my job was like that 
(Edit: is the one in the middle sulfur trioxide-pyridine complex?)
[Edited on 19-10-2013 by Mailinmypocket]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yep, mailinmypocket got it. I was doing a Parikh-Doering oxidation (non-cryogenic version of Swern, ideal for scale up). Input material was 1.4kg
alcohol substrate in each flask (the upper picture shows the workup of the reaction, which took 3 days in total). These pics were taken a few months
back, but that was 2 batches of 12 required for the custom manufacture campaign. Unfortunately the smell ofdimethyl sulfide byproduct progresses
throughout the next three manufacturing steps.
They're 20L rbfs btw ;p Also note how we don't shake the sep funnels at that scale. They remain stationary... The phases are mixed in the reaction
pot, transferred to the funnel by vacuum line and separated. Organic gets sucked/poured back into the pot and washed with the next solution.
Just another picture to keep people happy... Again in a 20L pot, equipped with a 2L addition funnel full of acid chloride solution. It feels quite
funny looking at these pictures now because I'm currently working on microscale reactions.

[Edited on 19-10-2013 by DJF90]
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
copper acetylsalicylate
<img src="http://chem.pieceofscience.com/wp-content/uploads/2013/10/copperII-aspirinate.jpg" width="600" />
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image size(s)]
[Edited on 21.10.13 by bfesser]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Beautiful. Nice work, <strong>Hegi</strong>.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

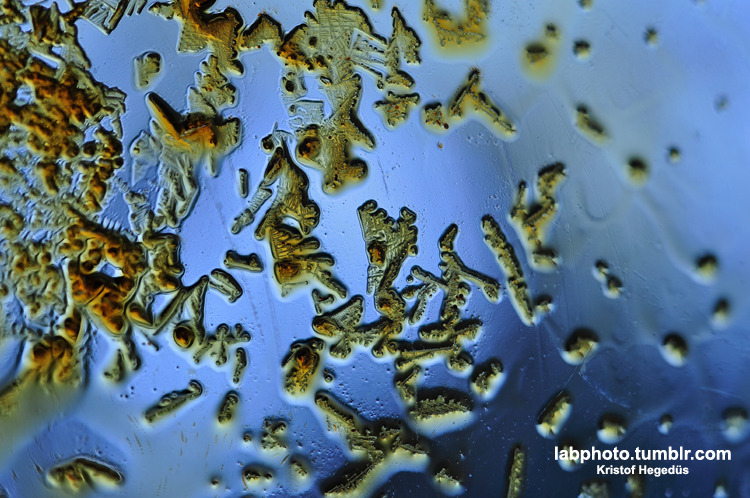
Some nice crystals which formed at the bottom a flask.
It was a polymethoxy furane deriative what formed as a side product of a reaction, but it will be really useful for me in the future.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
thanks, I will also post a picture of bis(oxalato)cuprate(II) tetrahydrate 
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
I love my tritium keychain! Maybe not "pretty" but really neat to always see the thing glowing after almost 8 years 
Description
<img src="http://i1329.photobucket.com/albums/w541/mmpchem/photo_zps608034b3.jpg" width="600" />
[Edited on 27-10-2013 by Mailinmypocket]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image size(s)]
[Edited on 27.10.13 by bfesser]
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
To satisfy large demands from the Vatican I decided to make isopropyl nitrite 

the bottom layer is really cool it looks alive and rippling
Shortly after this my stirring failed due to the Na2SO4 slush
I am really preparing this in preparation to distilling HCN as an antidote, just in case...
[Edited on 30-10-2013 by Pyro]
all above information is intellectual property of Pyro.  |
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Talking large scale? Talking hundred liters 

Edit: Sorry it's not very pretty.
[Edited on 10/30/2013 by BromicAcid]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Pyro  | To satisfy large demands from the Vatican I decided to make isopropyl nitrite 
the bottom layer is really cool it looks alive and rippling
Shortly after this my stirring failed due to the Na2SO4 slush
I am really preparing this in preparation to distilling HCN as an antidote, just in case...
[Edited on 30-10-2013 by Pyro] |
Yeah...I did the exact same reaction like a week ago. Modified orgsyn prep? Mine also failed when my stirring choked on sulfate. They are not kidding
about mechanical stirring. I had 3/5 of the sulfuric/alcohol in at that point too. I still scraped 1/2 of the supposed yield of ester though so it
wasnt a total wash.
I'm gonna nitrosate some cyclohexanone with it.
[Edited on 10-30-13 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
It was not my reaction, but: something crystallized from the mother liquor of an aromatic alkyl imidate and it looked great, just like on the
pictures. Organic crystals are awesome!

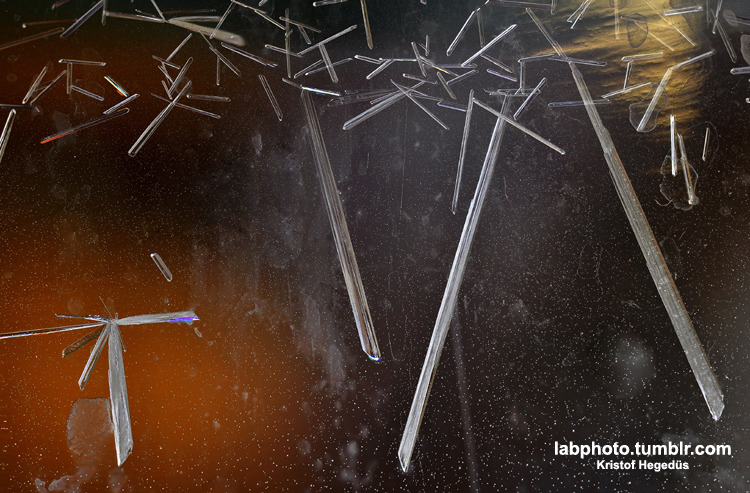

Have You seen my portfolio? It’s over here: http://labphoto.tumblr.com/tagged/portfolio
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nice setup Bromic! Thats a thick stirrer shaft on there... Thats what, 20-25mm dia?
Good to see all the secondary containment too!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
This was an attempt to grow crystals of copper(II) anthranilate by allowing methyl anthranilate to hydrolyse slowly in a solution of copper(II)
acetate in 50% aqueous acetic acid. They look more like copper(II) acetate to me, and seem to be somewhat soluble (which copper(II) anthranilate
isn't). So I'll chalk that up as a failure, along with the attempt to hook the microscope up to the computer so that I could get a picture without
resorting to an iPhone.
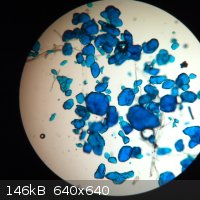
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
2
3
4
..
77 |