| Pages:
1
2
3
4 |
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Corrosive, volatile, fumes....
Hydrogen fluoride, boron trifluoride, boron trichloride, fluoboric acid, methyl triflate, triflic acid, iodomethane....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
dasgoose21
Harmless

Posts: 7
Registered: 28-8-2012
Location: Saturn
Member Is Offline
Mood: Ferrocious
|
|
Possibly oleum? If it fumes horribly, then this would fit because if pure SO3 Crystals were put in reagent grade H2SO4, then it could look like this,
but still fit every description
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Hydrochloric acid? Hydrogen cyanide?
[Edited on 11-4-2013 by ItalianChemist]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Almost every compound mentioned is colourless, transparent and liquid. This doesn't fit to the description. It has to be either solid or gaseous or
coloured/opaque/white in it's usually known state.
It's bromine. Colourless bromine.
[Edited on 11-4-2013 by Pok]
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Well he said it is not a liquefied gas nor a molten solid so I am thinking it has to do with the color. Perhaps it is a fluorescent or phosphorescent
liquid but under the lighting conditions it looks colorless.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
May be you have adjusted the lighting conditions so that it will appear colorless, when under normal lighting it has color?
So yea, it might be bromine or something of that sort.
|
|
|
Boron Trioxide
Harmless

Posts: 42
Registered: 18-6-2012
Member Is Offline
Mood: No Mood
|
|
Fluoroantimonic acid 
Perhaps it is liquid very pure white phosphourus in inert atmosphere, the melting point is supposed to be 44.2oC which could qualify.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
I thought of WP too. but it's boiling point, not MP.
[Edited on 12-4-2013 by Pyro]
all above information is intellectual property of Pyro.  |
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
is it bromine ? under a funny wavelenght of light making it look transparent
yeah i am with Mixell on that one
[Edited on 12-4-2013 by neptunium]
[Edited on 12-4-2013 by neptunium]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also go with mixell and neptunium. I have the impression that bromine does not absorb light at infrared frequencies (the tail of non-absorption
extends a little in the visible range, hence the dark red color of bromine). So, if endimion17 has used an infrared camera or adjusted a normal camera
by removing the IR-filter which is in many cameras, then I can imagine that he can make a movie and a picture like this.
Bromine is corrosive, toxic, gives dense fumes in air (not really fumes, but dense reddish gas) and if sufficiently pure and dry does not wet glass
and has a high density (well over 3 grams per liter).
Your background picture is a slight hint. It shows a frequency range of 4000 waves per cm to 800 waves per cm. This is in the IR and far IR range
(2500 nm for 4000 waves per cm to 12500 nm for 800 waves per cm). So, I consider this as extra evidence for my hypothesis that this is bromine imaged
at IR wavelengths.
[Edited on 12-4-13 by woelen]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'd go with bromine too.
C'mon, Endi, you've had your fun! 
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
The winner is Pok. 
Yes, it is bromine. I hoped no one will guess before I make another video, but it was kind of inevitable once people started talking about the
properties other than "what you see".
So this is how it actually looks like. 
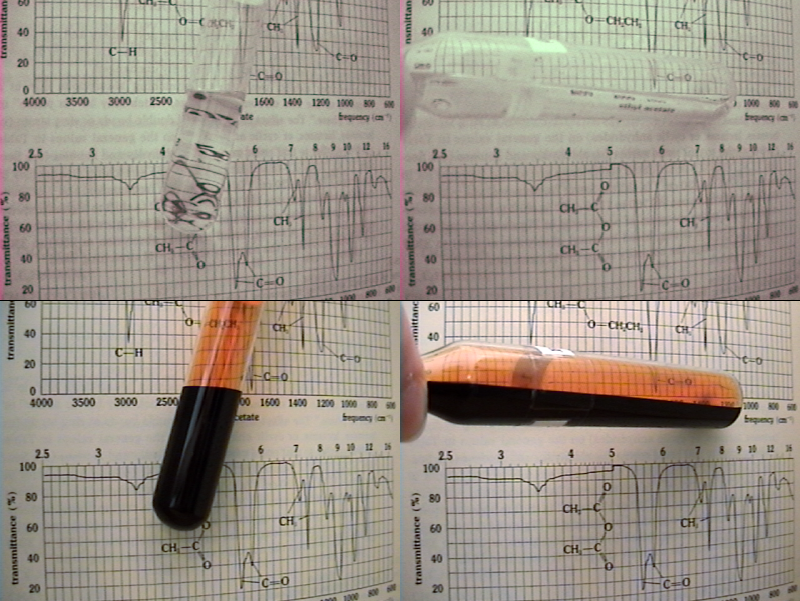
In the video, I've blasted the sample with sunlight and then, by combining a two set of filters and a camera lacking a "hot mirror" (otherwise it
won't work), removed everything but the >800nm range.
I often thought about this experiment, but I lacked equipment. Thanks to one guy ordering it for me, I was finally able to do it.
Talking about "colour" in the IR photos is futile. Although most photographers digitally enchance their photos, adding weird colors, it's not even
black&white. It's like trying to describe a temperature of 25,000 °C.
Our sensory apparatus is useless outside its evolved ranges, so the simplest thing is to convert it grayscale.
In the 700-900 nm range, bromine is completely transparent. I was really stunned the first time I saw it, given the fact we're used to look at it as
an optically extremely dense liquid. Also, to test a prediction made years ago was very satisfying.
woelen, you've really made a nice deduction there.  You're the only one who has
recognized the infrared spectrum table behind. It was one of the clues. Nice work. You're the only one who has
recognized the infrared spectrum table behind. It was one of the clues. Nice work. 
I've tested iodine, too. My hypothesis was that it would behave similar to bromine, but it turns out its melt is optically dense in this spectrum. It
appears black. Maybe it's because its molecules are so large and prone to electron cloud distortions while bumping close together, I don't know. Maybe
the range of transparency is shifted towards even greater wavelengths. I don't have the equipment to check it out.
What I predicted correctly is that its vapor is transparent. That nasty, thick vapor becomes invisible.
Now I'm in the range of making a small theory. If they're transparent in the gaseous phase, where the molecule interaction is poor, it might be that
they're really distorting each other in the liquid phase, strongly attenuating pasing IR. Bromine is a lot smaller molecule, so this bumping doesn't
distort it enough to affect its transparency in this wavelength range. I suppose liquid chlorine and fluorine would also be transparent.
What was interesting to watch is solid iodine. It is opaque (meaning black, in grayscale), but if you make highly ordered crystals, it is transparent.
It's notoriously difficult to make a large monocrystal of iodine (it tends to make lots of differently positioned layers) so only the thinnest
crystals look transparent. In transmitted visible light, iodine is pretty much like bromine, kind of red.
I'm making a video this afternoon, showing the transition for bromine, and I'll also test what happens with solid bromine. Something tells me it's
going to be transparent because it crystallizes in large grains. I think it will look like ice slush.
The move after that is to record a nice, clean video of iodine melting, something previously impossible to me, because of the optically dense vapor in
the visible range that makes dumb, uninterested teachers lie to kids about "iodine not having a melting point". Bromine also sublimes, but because its
vapors aren't so dense, we see it melting.
I think these things should be more recognized because the worst thing in teaching science is this dull, unimaginative anthropocentric viewpoint. The
goal should not be to relativize everything (that would be even more stupid), but to show why and when something is the way it is.
Anyway, people would've guessed it before if I haven't corrected the color of the IR shot. It was greenish, the tell tale of infrared shots.
[Edited on 12-4-2013 by Endimion17]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Very nice work. Beautiful to see that transparent liquid with the distorted image of the paper behind it, knowing that it is bromine.
This technique may also be very interesting to observe colorless or very weakly colored chemicals (e.g. gases), which cannot be seen in normal light
with our eyes, but which may become visible as dense liquids or fumes in the IR-range. Lots of new possible experiments! Keep up the good work!
[Edited on 12-4-13 by woelen]
|
|
|
Finnnicus
Hazard to Others
  
Posts: 342
Registered: 22-3-2013
Member Is Offline
|
|
Wow, wow, wow! That's really good reasearch there mate. Very excited for the iodine video. This was incredibly interesting, thanks. Could this effect
be shown with non-halogens?
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Really great! This may give us a small insight into the perception of e.g. animals that have a different range of wavelenght that can stimulate their
nerve cells. For bees this would look similar (but maybe coloured). Bromine should be more transparent for them because they can see infrared light.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by woelen  | Very nice work. Beautiful to see that transparent liquid with the distorted image of the paper behind it, knowing that it is bromine.
This technique may also be very interesting to observe colorless or very weakly colored chemicals (e.g. gases), which cannot be seen in normal light
with our eyes, but which may become visible as dense liquids or fumes in the IR-range. Lots of new possible experiments! Keep up the good work!
[Edited on 12-4-13 by woelen] |
Thanks. One of the things I've also tested is water. As predicted, is attenuates this range slightly, making a glass full of it look like there's a
grayish, transparent substance inside.
Gaseous chlorine - completely transparent as predicted.
I've been thinking about the same experiments - making previously transparent stuff look opaque, but I can't think of anything at the moment that
would be readily available outside my lab. Water vapor absorbs in even longer wavelengths...
If someone could test these things (and add some actual numbers to make it quantitative work) that would be great.
Quote: Originally posted by Finnnicus  | | Wow, wow, wow! That's really good reasearch there mate. Very excited for the iodine video. This was incredibly interesting, thanks. Could this effect
be shown with non-halogens? |
Yay! 
Liquid oxygen absorbs in the red part of the spectrum, so it might look pretty opaque in this, NIR (or IR-A) spectrum. Unfortunatelly I can't test
this.
Molten sulfur should be interesting, having all those colorful transitions, becoming deep red. It would probably glow, being that hot, but also
probably semi-transparent. Like a glowstick, perhaps.
Quote: Originally posted by Pok  | | Really great! This may give us a small insight into the perception of e.g. animals that have a different range of wavelenght that can stimulate their
nerve cells. For bees this would look similar (but maybe coloured). Bromine should be more transparent for them because they can see infrared light.
|
Insight, yes, but very small. We can find out whether they recognize the radiation, but their perception will probably be out of our league, forever.
Colour is an impression, a synthesis of inner workings of sensory organs and the central nervous system.
For example, people often talk about dogs seeing or not seeing some color. And they name it, thinking that the animal really sees what we see.
No one knows how do they perceive it. We might only say they recognize some wavelengths we perceive as green or whatever.
It's one of the things that makes me kind of sad. The number of wavelengths is infinite, and we only recognize few categories of color (and their
tints). There are infinite colour impressions out there, and we'll never experience them.
Take a look at this funny comic.
|
|
|
Finnnicus
Hazard to Others
  
Posts: 342
Registered: 22-3-2013
Member Is Offline
|
|
Liquid sulfur sounds awesome! How about metals? Or salts? Or am I misunderstanding?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, good work. I'm green with envy, truth be known! 
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
No. Its possible. Silicon (metalloid) is transparent in the IR above 1100 nm or so. Endimion17, do you have silicon and can you achieve this (>1100
nm) ?
http://www.flickr.com/photos/imager/3380554807/lightbox/
Or IR-transparent germanium: http://www.youtube.com/watch?v=nzoq4WjnfVA from 0:55
Some salts do behave similar (e.g. thallium bromo iodide - KRS5, which is dark red transparent in reality).
[Edited on 12-4-2013 by Pok]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I second that. I've often wondered/postulated what the world would look like to bees, butterflies (and fictitious species such as orcs and Klingons,
who are also suggested to have different visual ranges), and I'd love to be able to take pictures at such wavelengths.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
everything looks very different in the IR spectrum...i have dark brown eyes in the visible spectrum but greish blue eyes in IR!
also the opposite is true ...transparent glass in visible wavelenght is completly opaque in IR....
fascinating ! thanks for putting this out and for the idea Endi..
|
|
|
Finnnicus
Hazard to Others
  
Posts: 342
Registered: 22-3-2013
Member Is Offline
|
|
I'm not sure I understand (always learning), in the photo, red/bromine colored wavelengths have been removed? If this is true, does everything have a
particular set of wavelengths associated with it?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Metals should be completely opaque to any IR part because they're basically a cage of cations immersed in a gas made of electrons. Of course, if
heated well enough, just as any other approximated black body, they will emit some of it. But that's emission, not transmission.
Some salts are definitely transparent and are made especially for this purpose. As Pok said, thallium compounds. Halogenides, even combined
halogenides. I don't have any of them.
I don't have any germanium nor silicon to check it out.
However, not all IR is the same. I'm taking images in NIR, and "thermal cameras", the ones with psychodelic false colours, use lower frequencies. I
don't have such camera. They're very expensive.
Quote: Originally posted by neptunium  | everything looks very different in the IR spectrum...i have dark brown eyes in the visible spectrum but greish blue eyes in IR!
also the opposite is true ...transparent glass in visible wavelenght is completly opaque in IR....
fascinating ! thanks for putting this out and for the idea Endi.. |
No, just greyish. 
There are no perceptible colours above deep red. Some cameras with "nightvision" give off greenish image, but I think that's just a simple way to help
us see the details on the screen/goggles because eyes are most sensitive for yellow-green.
There are several confusing systems for categorizing IR, but to simplify, NIR crosses sodium and borosilicate glass without problems. My windows and
test tubes are completely transparent to it.
Sodium glass is very opaque in the MIR-FIR (middle and far) range, which is completely out of my league.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by Finnnicus  | | I'm not sure I understand (always learning), in the photo, red/bromine colored wavelengths have been removed? If this is true, does everything have a
particular set of wavelengths associated with it? |
Bromine looks black because it absorbs pretty much everything. Even red is absorbed so strongly it takes a thin layer or very strong lightsource to
shine through bromine.
It transmits the stuff below red quite well, such as near-infrared. So if you take a camera that registers NIR, and you shine NIR through the sample,
and use a set of filters that block any visible light, it will appear transparent.
I've taken these photos this afternoon.
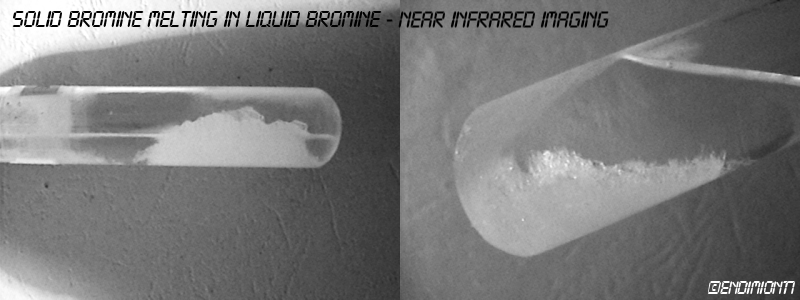
As you can see, bromine is completely transparent to NIR even when solid. It appears white (except the largest crystals) because of scattering,
similar to any white compounds such as sodium chloride.
In visible light, bromine is deep red on transmission. Its molecules are ordered, so its overall transparency increases, although certain frequencies
are efficiently removed (green, blue, etc.)
Just to add that, on reflection, bromine looks metallic. It's similar to iodine, basically.
[Edited on 12-4-2013 by Endimion17]
|
|
|
Finnnicus
Hazard to Others
  
Posts: 342
Registered: 22-3-2013
Member Is Offline
|
|
Ah so it is really dependent on the substance, not how it looks. Bromine's behavior under NIR is somewhat unrelated to the evil red color?
Could this be done with mercury? Why/why not? How would I found out for myself?
|
|
|
| Pages:
1
2
3
4 |