| Pages:
1
2
3 |
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Classy, nice.
There appears to be something in the actinium box, but it is difficult to see in the picture. What is the sample? Similarly, what is your Po sample?
it doesn't look like an atistatic brush.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
it is ! just the strip tho in a small glass vial. Actinium is just a sample of Euxenite...i made a gamma spectrum of it and Actinium is clearly
present
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
well, wouldn't it be nice if we all had a gamma spectrometer? LOL
I have a euxenite specimen, will there always be Ac present? I assume it is a decay product. is there any way I could verify Ac presence in a sample
without popping into my local university? its not an area I am very up to date on.
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=27963&...
send me a sample
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by diddi  | well, wouldn't it be nice if we all had a gamma spectrometer? LOL
I have a euxenite specimen, will there always be Ac present? I assume it is a decay product. is there any way I could verify Ac presence in a sample
without popping into my local university? its not an area I am very up to date on. |
Any sample of a natural uranium mineral will contain an equilibrium amount (i.e. same decays-per-sec as the parent U-235) of Ac-227. U-235 decays into
Ac-227 (half life 21.77 years) in a three step process with Pa-231 the slowest link (32,760 years) - so Pa-231 is also present in an equal-decay
amount (but over 1,000 times more by mass). Confirmation through gamma spectroscopy is interesting, but hardly necessary.*
Natural uranium ore specimens allow you to accurately claim all (but maybe one) of the members of the decay chain, there will be at least some of
every one of them, even the super-rare francium if you have at least a milligram of uranium in the sample.
The super-super-rare astatine I am not sure about - all of the production pathways are rare, and no good estimate of the likely content is at hand.
I'll have to work that one out a bit.
*It may set-up a nice sideline selling radioactive samples with their accompanying gamma spectrograms.
[Edited on 24-12-2014 by careysub]
[Edited on 24-12-2014 by careysub]
Attachment: U235.svg (94kB)
This file has been downloaded 794 times
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
If someone did that, I certainly would be interested. Bagging a bunch of the unobtainables for the element collection would be a coup.
edit
I still would like a gram or two of depleted uranium though. Having evidence of some decay products would be great but having a visible sample of the
great bogeyman element is still desirable.
[Edited on 24-12-2014 by j_sum1]
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
@Np
I have quite a few minerals: pitchblende, monazite, samarskite, ellsworthite, polycrase, davidite, autunite, allanite, thorite, fergussonite,
uraninite, aeschinite, euxenite, trinitite
and possibly some others I cant remember right now 
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Although it would require extensive planning to avoid contaminating the lab, for an element collection it would be nice to extract the various radio
elements into separate samples, so one can put them in separate boxes of a periodic table (even if they remain 'diluted' with stable elements, eg.
radium salt in a matrix of the barium salt, polonium in a bismuth fraction, actinium in a rare earths/lanthanum fraction, etc.)
[Edited on 24-12-2014 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
it is the original idea i had a while back...this periodic table is 20 some years in the process... time and money are a major issue sometimes.
i was going to print out the spectrum and glue it to the back of each compartment with a little arrow pointing at the peack responsible for (fill in
the blank) proving its presense.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by neptunium  |
it is the original idea i had a while back...this periodic table is 20 some years in the process... time and money are a major issue sometimes.
i was going to print out the spectrum and glue it to the back of each compartment with a little arrow pointing at the peack responsible for (fill in
the blank) proving its presense. |
You could offer this service to SM members for a reasonable consideration to offset the cost...
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
does anyone have access to a gamma spectrum library? I have bee googling but turning up nothing.
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
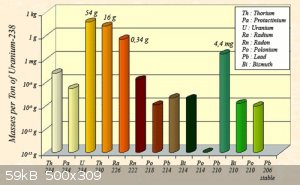
I found an interesting graph and text pertaining to abovementioned "radioactive equilibrium" here:
http://www.laradioactivite.com/en/site/pages/Radioactive_Equ...
the graph for U238 is nice:
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i did
http://www.sciencemadness.org/talk/viewthread.php?tid=27963#...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Seeing this thread bumped encouraged me to post an update. It also made me realize I really need better lighting for my display area! Getting half
decent pictures was tough.
Here's the state of the table as of today. The only elements I have left to collect are As, Rb, Pd, Nd, Lu, Hf, and Ir.

After those, there are several samples I'd like to improve. Blogfast's work with light bulb filament support wires has thrown doubt on my Mo sample,
for example. Also all elements from Po to Pa are currently represented by uranium ore samples, so at least some of these could be better (like Th).
On the table the display rests on, I have some extra neat things that either won't fit on their shelves or aren't really elements.
From left to right: Teflon cylinder, uranium doped marble, aluminum ingot (melted from soda cans), just over 1 kilogram (1061g) of mercury, white
phosphorus stored under water, lithium slugs under argon, and an ultra pure silicon crystal boule.

And then some gold ore samples and a fluorite octahedron in the left picture, and my mini collection of sulfide minerals on the right. The two
unlabeled ones are galena at the top and molybdenite on the bottom right.
 
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
This is ultra cool. I am really excited to see how it has progressed.
I saw some Ir on ebay recently at a good price. U2U me if you want details.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Thanks! It's been lots of fun filling the shelves. U2U sent.
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
  
only 6 more to complete it !..
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Nice, thanks.
Is there anything that physically prevents the bromine flask from rolling out of its box?
[Edited on 27-4-2015 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Awesome! What all do you have left to collect? What is in your polonium vial?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by MrHomeScientist  | Very recently I finally completed a pretty massive project that I thought I'd share with you all: a large periodic table display for my element
collection! I designed and built this myself, since my element collection has grown large enough where I think it deserves a nice display unit.
Broad overview: it is a large freestanding display unit with individual acrylic shelves for all 118 elements (plus two placeholders for the lanthanide
and actinide series'), with the shelves backlit by LED strips. The display is able to be broken down into four pieces, to allow for easy transport.
The LEDs are controllable via an Arduino "master controller" and several LED driver boards mounted to the back, allowing for some really neat effects.
In the future, I plan to write a tablet app that can control the display via a periodic table GUI. That will enable things like Quiz mode, where
multiple choice questions can be asked, the answers lit up on the board, and the correct one flashing (or something similar).
I put together a video summarizing the build and showing it in action:
<iframe sandbox width="560" height="315" src="http://www.youtube.com/embed/LSY_DKQv4g0" frameborder="0" allowfullscreen></iframe>
I go into much more detail on my blog, in a series of posts starting with the introduction: http://thehomescientist.blogspot.com/2013/02/the-element-dis...
I won't attempt to replicate all that info here, but here are a few fun facts about the display:
- Design time: 3 months
- Construction time: ~1 year (working during my free time)
- Dimensions: 48" tall x 65" wide (about 4 ft x 5.5 ft)
- Number of holes drilled: 361
- Cost: ~$1000 (not including the actual elements, or all the prototyping materials I went through)
- Current collection: 51 pure elements, 10 elements represented by radioactive decay products (U ore), and 2 representative compounds (Teflon and
AmO2)
Soon I'll be going through my whole collection in another set of videos as well, so stay tuned for that! |
Dude!
You sooooo nailed it.That is just amazingly cool.
You know... I can easily see 700.00 - 1,300.00 bucks for these retail.
That's a Shark Tank episode waiting to happen.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
szuko03
Hazard to Others
  
Posts: 188
Registered: 3-4-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
"That an amazing idea I wish you all the luck but it's too niche for me, I'm out."
But ill probably be borrowing from your idea in a decade or so. Ill try to give credit because it's way too amazing for me to claim.
[Edited on 27-4-2015 by szuko03]
Chemistry is a natural drive, not an interest.
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Quote: Originally posted by phlogiston  | Nice, thanks.
Is there anything that physically prevents the bromine flask from rolling out of its box?
[Edited on 27-4-2015 by phlogiston] |
nope...just the thick glass ampoule!....and carpet!
its fine it hasnt rolled off yet
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
static brush from static master..
http://www.amazon.com/Static-Master-Brush-1-Inch/dp/B0000AE6...
the strip has a pretty god Po210 source in it..
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have heard that before -- probably from Theo Gray. Thanks for the reminder.
(Po is probably one of the lower priorities for my collection however. I should do a count, but I am only about half way with all of the expensive
ones to go.)
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Awesome! Pretty expensive, plus you have to replace it every ~470 days! (the time I calculated it to decay to 10% remaining Po) I'd love to get one of
those. If it's possible to cut off a piece of that strip I'd be willing to trade for it 
I have my bromine sample similarly precariously placed on its shelf. "It hasn't fallen off yet" won't sound so good after it does fall off! Ideally
I'd like to encase mine in acrylic, but in the meantime maybe some sort of base/stand for it would be a good idea. In your case, you could put a dowel
of some sort across the 'cubby' to keep it secured.
Thanks very much for the praise on my table, by the way  The display plus all
the samples would end up being pretty expensive if I were to turn it into some sort of product; the samples probably double the overall price. Now
that I've taken it to several events, I'd really like to sit down and redesign it to be more sturdy. More like a cabinet, with windowed cells so the
samples are secure in their places and people can come right up to the display without fear of knocking anything over. The compartments would open
from the back so I could still take samples out to show people. Something like that, anyway. The display plus all
the samples would end up being pretty expensive if I were to turn it into some sort of product; the samples probably double the overall price. Now
that I've taken it to several events, I'd really like to sit down and redesign it to be more sturdy. More like a cabinet, with windowed cells so the
samples are secure in their places and people can come right up to the display without fear of knocking anything over. The compartments would open
from the back so I could still take samples out to show people. Something like that, anyway.
|
|
|
| Pages:
1
2
3 |