| Pages:
1
2
3
4 |
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
CrO3 when mixed with ethanol reacts vigorously to form Cr (II, III).
I got a small crucible I'll give it a try for sure making some of it.
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
I tried producing the chromium trioxide by adding anhydrous sodium dichromate (dried in the oven: at first you may say "omg it caked", but as it
slowly cools it easily releases from the glass) to 98% sulfuric acid. It dissolved, warming the vessel. I did not measure but I would put the
temperature around 60°C, was just hot to touch, guess it could be heated even more au bain marie. Also realised the more excess sulfuric acid you add
turns the mess into a better oxydising paste, no better than actual aqueous solution of both ingredients  . After cooling it becomes a dark red mud. . After cooling it becomes a dark red mud.
Analysing the reaction
H2SO4 + Na2Cr2O7 --> CrO3 + Na2SO4 + H2O
The problem obviously is separate the sulfuric acid and water from the rest of the contents. CrO3 decomposes at 251°C, while sulfuric acid boil at
more than 300°C. So a vaccum pump mut be put to work as to easily get rid of the sulfuric acid.
Again I insist useing ammonium componds as the product of the dichromate because the procuct ammonium sulfate can evaporate too.
For the synsthesis of ammonium dichromate, do as follows, it has been succesfully tested:
(I) Na2Cr2O7 + 2 NaOH --> 2 Na2CrO4
(just add solid NaOH to conc. solution of dichromate)
(II) Na2CrO4 + CaCl2 --> CaCrO4 (ppt) + NaCl
Make a ten-fold excess solution of calcium chlorid so as to pour the conc. sodium chromate, this will render a lower degree of sodium contamination
and also ensure reaction will dislocate as to precipitate CaCrO4 by means of common ion effeft. To this point CaCrO4 precipitates as a hydrate.
Filter. Throughly dry in the oven, at 180°C. Now, the anhydrous salts may be washed with boiling water: very few of the now anhydrous CaCrO4 will
dissolve, while all other salts will be taken away.
next
(III) 2CaCrO4 + 2(NH4)HSO4 --> (NH4)2Cr2O7 + 2CaSO4
Prepare a saturate solution of ammonium bisulfate at 80°C, pour the anhydrous CaCrO4 and stir to dissolve. This can be next diluted to facilitate
filtration.
Now you have the precious ammonium dichromate!

Attach: sulfuric acid vapour pressure graph
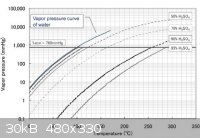
[Edited on 8-31-2012 by Poppy]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Is there any OTC source for ammonium bisulfate?
Once I have the ammonium dichromate, what do I do with it?
EDIT: CrO3 -(197+ C)> Cr2O3 + O2
Why isn't this feasible?
[Edited on 31-8-2012 by elementcollector1]
[Edited on 31-8-2012 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
The ammonium dichromate carries the chromium content you dealing with all the time!!!
Its a bet you can achieve the high purity light green Cr2O3 and then experiment with CrO,
H3PO2 + 2 Cr2O3 → 4 CrO + H3PO4
Then check what wiki means about the decomposition of CrO at 300°c
Also, check the sticky on the preparation of elemental phosphorus!
Good luck!
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I read somewhere on this forum (thread was complete with pictures) that the decomposition of ammonium dichromate does not produce pure Cr2O3, it
instead produces a rather dark green powder ripe with impurities
(Ze search function fails me yet again, I can't find ze thread...)
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
Yeah probably the thread is somewhere, couldn't find it either.
The decomposition method yields a low purity Cr2O3 powder for which there is no much description regarding its behaviour against acids.
I would dream of an equation where CrO desproportionates to Cr and some other chromium oxide!
like
3CrO --> Cr + Cr2O3 !!!!
This could then be purified useing CO to form carbonyl complexes out of the metallic chromium, leaving the oxides unreacted, while Cr(CO)6 might be
solvated and extracted 
[Edited on 8-31-2012 by Poppy]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Washing with distilled, boiling water would probably improve the quality of the chromium oxide, because that would remove all soluble compounds like
unreacted dichromate, sodium/ammonium compounds, etc, leaving behind only a mix of chromium oxides (CrO, Cr2O3, and CrO2).
This would probably still work for a thermite.
I've seen several videos of the ammonium dichromate decomposition, and while it is insanely cool, I would probably prefer to just decompose the CrO3
(unless it's an explosion or something).
Is there a reaction for the decomposition of sodium dichromate?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
No no, sodium holds everything together for longer. It turns into a melt, and stays like that, specially the dihydrate.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Hmm. Well, how do I turn sodium chromate to pure sodium dichromate? Then I can start the conversion to CrO3, and probably decompose from there.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Forget about decomposing ammonium dichomate thermally, it's not great for pure Cr2O3.
Instead dissolve it in water, add some acid and add (slowly!) methylated spirits, then gently heat if necessary. The alscohol reduces the Cr (VI) to
Cr (III) (green/blue). Now alkalise the solution with ammonia and Cr(OH)3.nH2O precipitates. Filter this off, wash with copious amounts of water and
dry/calcine to Cr2O3.
Instead of alcohol, hydrogen peroxide can also be used.
[Edited on 1-9-2012 by blogfast25]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Er, has anyone heard what I've said? About CrO3 decomposing to Cr2O3? I've gotten no feedback on that so far, and it would be great if I could just
skip most of the steps to that part.
Blogfast, what if I used sodium dichromate instead?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | Er, has anyone heard what I've said? About CrO3 decomposing to Cr2O3? I've gotten no feedback on that so far, and it would be great if I could just
skip most of the steps to that part.
Blogfast, what if I used sodium dichromate instead? |
CrO3? Basically I'd forget about that if I were you.
All dichromates reduce alcohols and H2O2: it's a function of the anion, not the accompanying cation (K, NH4, Na,...)
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Aww. I thought that might work, given how there would be no extra stuff in CrO3, just more oxygen.
Well, I switched to the chemical method a while back,and have a mix of my stainless steel chlorides and bleach reacting and filtering away. Last I
saw, it was just beginning to turn yellow.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
Could you describe what's that you performing with the ss you have, becaue a bunch of SS is sometimes difficult to dissolve
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
1. Dissolved the stainless in a bunch of concentrated HCl. After a while, the solution darkened from clear to emerald green to opaque green. No reflux
was needed, just some time.
2. Added bleach. Ferric hydroxide precipitated out in large quantities, this is busy being filtered out while the bleach additionally reacts with the
chromium (III) hydroxide precipitated to form sodium chromate.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
keep the acid
If your HCl goes weak you should attach a HCl generator lined into the batch vessel, the use of a non expensive silicon rubber tube should suit for a
couple runs
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Well, I got what I thought was a large quantity of sodium chromate, but upon adding acid it did not change color to the orange dichromate, so... Any
thoughts?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Poppy
Hazard to Others
  
Posts: 294
Registered: 3-11-2011
Member Is Offline
Mood: † chemical zombie
|
|
You aware how many acid you must put to displace the reaction? haha
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Not much: http://www.youtube.com/watch?v=zP9qEiaL4kQ
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
At a very minimum, check pH after acid addition. And if the chromate/dichromate concentration is relatively low the colour change may be subtle...
[Edited on 7-9-2012 by blogfast25]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
It's not even subtle, still at that same bright yellow. Unless that's iron?
Here's an idea, why not run electrolysis of the chromate. That would get rid of any iron present as the insoluble hydroxide, and in all likelihood add
more chromium to the solution from all that acid. Granted, I'll have to filter the mess again, but I'll boil it down this time to concentrate whatever
chromate is present. Is there any way to remove trace iron that somehow made it into solution? And why didn't the concentrated HCl have any effect on
the chromate?
I don't have anything in the way of pH checking.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
If it's iron it would have to be quite a lot to match the intense yellow of the CrO<sub>4</sub><sup>2-</sup> ion. Test for Fe
(III) with KSCN (red FeSCN<sup>2-</sup> and for chromate with lead
nitrate (yellow PbCrO4 precipitates) or silver nitrate (reddish Ag2CrO4 precipitates). and for chromate with lead
nitrate (yellow PbCrO4 precipitates) or silver nitrate (reddish Ag2CrO4 precipitates).
But going by your description there should be no iron insolution because Fe(OH)3 is extremely insoluble.
Check pH of your chromate/dichromate solution.
You can also check for dichromate as follows:
* to a small sample add an excess of clear 'denaturated spirits' (ethanol + methanol), warm up: the yellow should dissapear and make way for a light
green/blue (Cr3+)
* in the above substitute the alcohol with H2O2.
[Edited on 8-9-2012 by blogfast25]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Upon standing for three days, the solution now seems to have a metallic substance floating on top of it. I don't think this is metallic chromium, but
what else could it be?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
madcedar
Hazard to Others
  
Posts: 116
Registered: 10-9-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | I read somewhere on this forum (thread was complete with pictures) that the decomposition of ammonium dichromate does not produce pure Cr2O3, it
instead produces a rather dark green powder ripe with impurities
(Ze search function fails me yet again, I can't find ze thread...) |
Plante1999 killed some spoons here:
http://www.sciencemadness.org/talk/viewthread.php?tid=15839
Look at these too:
http://www.sciencemadness.org/talk/viewthread.php?tid=19202
http://www.sciencemadness.org/talk/viewthread.php?tid=13347
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Excellent! That second thread was the one I was talking about.
Anyway, some of that strange metallic float disappeared, but it still remains, oddly enough, in the already filtered flask.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
| Pages:
1
2
3
4 |