| Pages:
1
2 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Earth to AndersHoveland , earth to AndersHoveland ,
you're not making sense ,
circulate your air through the CO2 scrubber.
_______________________________
www.sciencemadness.org/talk/viewthread.php?tid=9486#pid11027...
Trichlorotriazine (TCT) is not TCCA , alias
- Trichloroisocyanuric acid
- 1,3,5-Trichloroisocyanuric acid
- 1,3,5-Trichloro-S-triazine-2,4,6-trione
- 1,3,5-Trichloro-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
etc. etc.
While admittedly 2,4,6-Trichloro-s-triazine has a positive heat of formation
http://webbook.nist.gov/cgi/cbook.cgi?Formula=C3Cl3N3&Un...
Anything that can be written on the right side of a balanced equation for this C3Cl3N3 =>
will have a more positive heat of formation.
We've been down this road before ,
www.sciencemadness.org/talk/viewthread.php?tid=17012&pag...
in this case TCT is the trimer of Cyanogen chloride ClCN , it is even more endothermic
http://webbook.nist.gov/cgi/cbook.cgi?ID=C506774&Units=S...
this cannot explode into something else because that will have again a more positive
heat of formation , why it instead condenses into the trimer. ( clue , hint , hint )
It's not too poor an oxidizer though and may make
something approaching a bang if finely mixed with a
suitable reducing agent such as cesium.
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Wouldn't it be cool to use NaHSO4 instead of acetic acid? It is stronger acid and you can get it pure (not 8% like vinegar) easily.
Rest In Pieces!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Adas  | Wouldn't it be cool to use NaHSO4 instead of acetic acid?
It is stronger acid and you can get it pure (not 8% like vinegar) easily. |
Why would this be cool ? When white vinegar is all that is called for
and only serves as a catalytic activator. " It is a stronger acid " which
means hypochlorous acid is formed instead of the hypochlorite ion.
See _
www.sciencemadness.org/talk/viewthread.php?tid=20296#pid2520...
Commercial grade sodium bisulfate is anything but pure containing
many impurities since it is intended to unclog drains , or adjust the
pH of a pool at enormous dilution.
.
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Ok..
I tried to make this .... I do get a fluffy white filterate but it will not ignite !!
Does it have to be absolutely dry ?
Damp filterate with a chloramine odour just melts and smokes.. No deflagration !!
I am itching to react it with some NaNO2 and hopefully (95% sure not gonna happen) and produce RDX. (That 5% chance is worth it)
Also wont mind trying it with some NaN3 soln and have a =N-N3 bond.
Chances of that happening seem higher than -NO2 as we have cyanurictriazide but no trinitrocyanauric acid .... Loosely speaking.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Must be very dry, and dry it fast. Mine decomposes even after a good wash into a yellow stain when left on the filter.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Thanks Bot0nist.
I shall dry it ASAP.
Anyone have access to NaN3 ? If you do .... Please try a few mg qty
I will try dissolving dry filtrate in anhydrous MeOH and adding a MeOH/NaNO2 soln.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I really do not think an azide group could be substituted onto a nitrogen atom. Reacting NaN3 with 1,3,5-trichlorohexahydrotriazine would most likely
just form diazomethane, or possibly a trimer thereof, in addition to liberating nitrogen gas. This would be rather dangerous.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/files.php?pid=260440&...
Just as the azidamine of methylene can reform into terazole ,
a trimer of tetrazole can result from 1,3,5-triazidohexahydrotriazine.
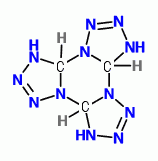
In the same way Cyanuric Triazide is isomeric with Benzotrifuroxan
www.sciencemadness.org/talk/viewthread.php?tid=4094#pid13473...
known organic azide derivations
http://www.sciencemadness.org/talk/viewthread.php?tid=217#pi...
________________________________________________
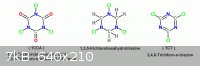
This post is a summary of the various ideas by forum members and myself on
the notion of substituting labile Chlorine in the above shown Triazine analogs
with an Azide or Nitro group by metathesis with an alkali salt such as Sodium
Azide or Sodium Nitrite. Considering each in turn.
TCCA
http://www.sciencemadness.org/talk/viewthread.php?tid=4282&a...
1,3,5-Trinitro-1,3,5-Triazine-2,4,6-trione , or triketo RDX is apparently according
to member PHILOU Zrealone a known compound
http://www.sciencemadness.org/talk/viewthread.php?tid=4282&a...
niertap unknowingly suggested the same thing here _
http://www.sciencemadness.org/talk/viewthread.php?tid=21453
The Azide variant has been unsuccessfully attempted by Formatik
http://www.sciencemadness.org/talk/viewthread.php?tid=21453#...
Solubility of TCCA in water is meager and enables hydrolysis of any resulting
product which is the central problem with producing what amounts to an
acid anhydride , necessitating use of non aqueous solvation.
Trichlorohexahydrotriazine
Axt first broached the present topic in this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=9370
The_Davster observed that =>
http://www.sciencemadness.org/talk/viewthread.php?tid=9370#p...
http://www.sciencemadness.org/talk/viewthread.php?tid=9370#p...
The reference cited from
Chemistry and Technology of Explosives vol 3 by Urbanski

TCT
The_Davster
" Not to get off topic, but the reaction of cyanuric chloride and nitrite was tried and failed ?
...fascinating, I had always wondered about that reaction."
http://www.sciencemadness.org/talk/viewthread.php?tid=9370#p...
The intended Trinitrotriazine has not resulted by this means , however
Cyanuric triazide is well known and readily prepared this way =>
http://www.sciencemadness.org/talk/viewthread.php?tid=4094#p...
Often the expected or rather hoped for result never does and something
unanticipated occurs instead. This paper notes different results occur depending
on the solvent and reaction conditions. For example TCT ( here called CC) with NaN3
Recent Applications of 2,4,6-Trichloro-1,3,5-Triazine & its Derivatives in Organic Synthesis
www.sciencemadness.org/talk/files.php?pid=94971&aid=3046
Carbon Nitride was synthesized by heating Cyanuric Chloride and Sodium Azide
in benzene at 220 ºC, which forms high quality nanotubes 14 ( Fig. 4 ) ,
by self assembly. Guo, Q.; Xie, Y.; Wang, X.; Zhang, S.; Hou, T.; Lv, S. Chem.
Commun. 2004, 26–27.
.
[Edited on 12-10-2012 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Here 's a thread that relates to TCCT , concluding there is
scant prospect of successfully obtaining the hypothetical
1,3,5-Trinitro-1,3,5-Triazine-2,4,6-trione
http://www.sciencemadness.org/talk/viewthread.php?tid=18690#...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
That's TCCA , sorry about that
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The unstability of NN'N''-trichloro-s-triazane is due to the presence of hydrogen fuel to burn aside on the carbons.
See the tread on chloramines by Axt.
The chlorine atoms are reactive chlorine atoms of the kind Cl(+) (due to the electronegativity of the N atoms), like what is found in hypochlorites
and chloramines.
HOCl --> HO(-) + Cl(+)
A contrario, cyanuric chloride displays chlorine atoms of the kind Cl(-) because they are fixed on a carbon and are thus exchangeable with negatively
charged anions (like OH(-) to get cyanuric acid)).
This might explain that nitrite anion can't subsitute easily the chlorine atom, but that HNO3 (what is a NO2(+) generator) is able to convert it into
nitramine.
One can think that nitroniums salts or F-NO2 will work because F is so électronegative that only NO2(+) can result. Also for the perchloryl one must
use F-ClO3
Maybe by inversion of polarity NN'N''-trifluoro-s-triazane may react with NaNO2 or NaClO3...
Maybe that AgNO2 (owing to AgCl precipitation as driving force) will lead to some conversion with NN'N''-trichloro-s-triazane but both reactants are
not very soluble and the solvent is the problem because it must be unreactive towards positive chlorine.
Also the nitrite-nitro rearrangement is 50/50 and if R2N-NO2 is stable R2-N-O-N=O shouldn't be...thus leading to unstable parts in the molecule...
with spontaneous decomposition reactions.
[Edited on 19-10-2012 by PHILOU Zrealone]
[Edited on 19-10-2012 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I just made a test, and it seams that the chlorine can't be substitued by nitrite. I made a solution of the 1,3,5-trichlorohexahydrotriazine in
methanol, and did the same with sodium nitrite. When mixed, the two solution did, well, nothing. They simply looked like a normal solution. I also did
a test with silver nitrite, without succes etheir.
A warning note for 1,3,5-trichlorohexahydrotriazine, I had stored a gram of it in a semi-closed vial for the night. When I came back in the moring,
the vial had closed for some reason, a pitch black material was on the bottom of the vial, and when opened, and hydrochloric acid smell was made.
Decomposition should look something like this:
2C3H6N3Cl3-) 6HCl + 3N2 + CxHx+y + yC
I never asked for this.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | | I just made a test, and it seams that the chlorine can't be substitued by nitrite. I made a solution of the 1,3,5-trichlorohexahydrotriazine in
methanol, and did the same with sodium nitrite. When mixed, the two solution did, well, nothing. They simply looked like a normal solution. I also did
a test with silver nitrite, without succes either. |
Most likely, the trichlorohexahydrotriazine just oxidized the nitrite to nitrate. Aqueous solutions of these N-Cl compounds act as oxidizers, because
of partial hydrolysis to hypochlorous acid (there is equilibrium in the solution). You would have to try the substitution reaction with a non-aqueous
solvent. (usually I would recommend DMSO, but I recently read that DMSO violently reacts with acetyl chloride, so this solvent may not work here)
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Anders he used methanol. But I don't get what one should expect from Cl+ + Ag+ --> ??? type reaction, as the chlorine is positive here. What if you
tried xxN-Cl + HONO2 -> xxN-NO2 + HOCL reaction, is there a chance for this to happen?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
It is not exactly my type of thing to try many reaction to make a new process for energetic material synthesis.
People where asking me to try it, so did I, but I think I completed my part of the conctract, finding nitrite, remaking the chloramine etc...
I never asked for this.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Ok,ok, I asked to see what the more competent people think about my suggestion, but if you have nitric acid you could try that (may be quite
dangerous, little amounts only). And if you have TCCA also to try it directly with nitric in the hope to get keto-RDX .
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
It might be prohibitively dangerous, but I have read reactions where the solution is heated, severing the N-Cl bond, and leaving radicals that can
then react. I will describe this references at the bottom of this post.
I am not sure if a R2N• radical could react with NO2 to make a nitramine.
It is more complicated than I care to explain, but there is more reason to believe this might work with trichlorohexahydrotriazine than with TCCA (and
even if it did work with TCCA, there would just be immediate hydrolysis to the free nitramine).
References:
R.A. Carboni, J.C. Kauer, J. American Chem. Society, volume 89, p2633, (1967) describes a reaction where an azide group on an organic
scaffold is decomposed, leaving behind a nitrogen radical that was then able to react the central (2-) nitrogen of a 1(N)-substituted 1,2,3-triazole.
What is remarkable about this is that the nitrogen atom becoming bonded was already in a 3-valence state. But just as tertiary amines can still be
oxidized to form the N-oxide, so too here was the nitrogen atom in the aromatic ring able to bond with an additional nitrogen radical.
In another reaction, N,N-dibromo-tert-butylamine, tBu-NBr2, was heated, driving off the bromines and leaving a nitrogen radical that was
able to react with another nitroso compound to leave an azoxy bridge.
tBu-N• + O=N-R --> tBu-N=N(-O)-R
(sorry, I do not have the exact reference for this reaction, this is just from my notes, I think it was A. M. Churakov published in
Tetrahedron), but I remember reading in a paper another investigator questioned the procedure as originally described by the original russian
investigators)
I think chlorine would also work instead of the bromine here.
In another investigation, they describe the preparation of dibromoamines in situ from tribromocyanuric acid and the amine. Obviously the HOBr
hydrolyzes off the TBCA and preferentially condenses with the amine, could be described as an equilibrium reaction. I just wanted to point out that,
even though it can be considered an "oxidation reaction", the bromine atoms are not actually oxidizing the amine.
Sorry, I do not like elaborating on long tangents, but I also want to preemptively address potential misconceptions and questions in a single post. It
can be frustrating having to explain everything later, and I would prefer nothing be misunderstood.
Also, if you are going to attempt N-Cl nitrite substitution reactions, I would recommend propylene carbonate as an ideal solvent, since it can
dissolve ionic compounds, and would also be unreactive here.
[Edited on 29-6-2013 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ plante1999
Examining the supposed reaction we see that the formation of NaCl is favored by
the enthalpy of reaction being negative. This is determined from the bond energies.
Starting materials total bond energies is 125 kcal _
Chloramine : H2N-Cl , 60 kcal
Sodium Nitrite : Na-ONO , 65 kcal
Reaction result bond energies is 139 kcal _
Nitramine : (CH2)2N-NO2 , ~ 41 kcal
Sodium Chloride : Na-Cl , 98 kcal
A catalyst seems to be called for to reduce the activation energy. The Problem
as I see it , is that NaONO is soluble as ions whereas the organic Chloramine is
solvated as a covalent molecule , so there is no basis for metathesis.
Electronic character of Sodium Nitrite , NaONO
Electronegativity Na = 0.9
Electronegativity -O = 3.4
the difference is , 3.4 - 0.9 = 2.5 > 2 , an ionic bond
Electronic character of Chloramine , =N-Cl , a covalent bond
Electronic character of Sodium Chloride , NaCl
Electronegativity Na = 0.9
Electronegativity Cl = 3.0
the difference is , 3.0 - 0.9 = 2.1 > 2 , an ionic bond
However _
Electronic character of , Magnesium Chloride , MgCl2
Electronegativity Mg = 1.3
Electronegativity Cl = 3.0
the difference is , 3.0 - 1.3 = 1.7 < 2 , a polar covalent bond
Electronic character of Magnesium Nitrite , Mg(NO2)2
Electronegativity Mg = 1.3
Electronegativity -O = 3.4
the difference is , 3.4 - 1.3 = 2.1 > 2 , an ionic bond ( although weaker than with sodium )
MgCl2 and Magnesium Nitrite are soluble in Methanol
The addition of some MgCl2 ( de-icing salt ) to the solution will procede as follows _
2 NaNO2 + MgCl2 => 2 NaCl + Mg(NO2)2 , , 2(-98 ) - (-154 ) = - 43 kcal
MgCl2 = ∆Hf -154 Kcal/mol
NaCl = ∆Hf - 98 Kcal/mol

Encyclopedia of the Alkaline Earth Compounds from page 210[/size][/font]
http://books.google.com/books?id=yZ786vEild0C&pg=PA210&a...
Magnesium Nitrite is not stable and re-formation of MgCl2 favors exchange
with the organic Chloramine. Mg(NO2)2 + 2 ( =NCl ) => MgCl2 + 2 ( =NNO2 )
Starting materials total bond energies is 102 kcal _
Chloramine : H2N-Cl , 60 kcal
Magnesium Nitrite : NO2Mg-ONO , 42 kcal
Reaction result total bond energies is 118 kcal _
Nitramine : (CH2)2N-NO2 , ~ 41 kcal
Magnesium Chloride : ClMg-Cl , 77 kcal
MgCl2 does form a complex with Methanol
Magnesium Chloride forms hydrates with 2, 4, 6, 8, and 12 molecules of water.
Both the anhydrous and hexahydrate salt are deliquescent and need to be stored
under dry cool conditions. Magnesium Chloride is very soluble in water 54.6 gm
100 ml, and the hexahydrate is the only stable hydrate between 0 and 100 °C.
The anhydrous salt is also soluble in Methanol ( 20.4 gm at 60 °C) and Ethanol
(15.9 gm at 60 °C). On cooling , these solutions crystallize the alcoholate addition
compound , such as Magnesium Chloride hexamethanolate , MgCI2 •6CH30H ,
and Magnesium Chloride hexaethanolate , MgCI2 •6C2H5OH
The Chemistry and Technology of Magnesia from page 216
http://books.google.com/books?id=0ShuV4W0V2gC&pg=PA216&a...
Heating MgCl2•6H2O yields the basic salt, Mg(OH)Cl
MgCl2•6H2O => Mg(OH)Cl + HCl + 5H2O
Pure anhydrous chloride can be prepared by heating the double salt
MgCl2•NH4Cl•6H2O
MgCl2•NH4Cl•6H2O => MgCl2•NH4Cl + 6H2O
Ammonium Chloride sublimes on further heating , leaving pure anhydrous MgCl2
MgCl2•NH4Cl => MgCl2 + NH4Cl
— Some observations _
An issue that is troubling is that the bond energy of Chloramine is 60 Kcal ,
19 Kcal more than the Nitramine bond of 41 Kcal , an endothermic substitution.
One can suppose that the solution temperature will drive the reaction.
The best solvents for RDX are Dimethyl Formamide , Acetonitrile , Acetone , in
descending order. RDX is sparingly soluble in methanol , so it and everything else
it seems will drop out of solution upon completion of the reaction , also driving
the reaction to completion.
Stability of Choramines is pH dependent. Acid low pH accelerates decomposition.
The problem then is that nitrous acid is formed from the nitrite salt and this reacts
with the Methanol to produce Methyl Nitrite. pH needs to be neutral or basic.
The application of a lewis acid such as Aluminum Choride , Zinc Chloride , does
facilitate breaking covalent bonds , but in the present case it will react first with
the ionic Sodium Nitrite just as well as the Magnesium Choride wil. But these
have a lower enthalpy of reaction and then inhibit the reaction from going forward.
Regarding other Nitrites , electronegativity of Silver Ag 1.93 and Copper Cu 1.9
exhibit pronounced polar covalency in their bonds. A low activation energy is the
likely reason Silver Nitrite at least has found success in this type of reaction. The
use of Copper in this scheme might be worth investigating , since Copper Nitrite
is not a stable compound , with Nitrogen dioxide faintly bound to it. Copper Sulfate
can be tried used instead of Magnesium Chloride.
Finally the use of solvent or more usefully a mixture of solvents is well known to
enhance or inhibit reactions. Dimethyl Sulfoxide is unsurpassed for aiding in the
placement of a nitro group onto an organic compound from a Nitrite salt. This
is so far only cited for carbon bonds , and is essentially a Finkelstein SN2 type
reaction.
.
[Edited on 29-6-2013 by franklyn]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | The Problem
as I see it , is that NaONO is soluble as ions whereas the organic Chloramine is solvated as a covalent molecule , so there is no basis for
metathesis. |
The N-Cl bond is weaker than the C-Cl bond. Chloramines can be protonated which leads to easy hydrolysis of the chlorine off the amine.
Trying to perform a substitution reaction in this situation has several potential problems, but getting the chlorine to come off is not really one of
them.
One investigation mentions that nucleophilic substitution on chloramine to form chloride ions proceeds at a rate more than 100 times that of
chloromethane (which may still be rather slow, considering that the hydrolysis of chloromethane with water takes around a whole year)
"Gas phase reactions of NH2Cl with anionic nucleophiles: nucleophilic substitution at neutral nitrogen", Roustam Gareyeva, Shuji Katoa, Veronica
M. Bierbauma
Organic Chemistry of Explosives, p234, claims:
| Quote: | | Metathesis reactions between N-chloramines and silver nitrite in alkaline solution are reported to give the silver salt of the corresponding primary
nitramine. |
There are some other threads in this forum about nitrogen trichloride, where you can read about how it reversibly hydrolyzes at low pH to ammonium
ions and hypochlorous acid. At higher pH, it can hydrolyze to chloramine, though such solutions can be unstable if not highly diluted. It is much too
complicated to try to get into the details of the reaction here, but the point is that the N-Cl bond can easily be hydrolyzed.
There has been similar discussion on this forum several times before. Typically what happens is some topic is being discussed, and then some
member brings up this idea of making RDX by metathesis with NaNO2 or AgNO2 . I have seen it happen at least 3 times before. Someone should really
start a specific thread for this idea.
I would suggest trying to react trichlorohexahydrotriazine with sodium nitrite in the absence of water, using propylene carbonate as
the solvent. If there is any water, the nitrite will just be oxidized.
Even using alcohol, there might be undesirable reaction. Methanol apparently seems to be fairly inert to TCCA, but I would not completely discount the
possibility of the solution containing some equilibrium of methyl hypochlorite, which could potentially be a reaction pathway for the oxidation of the
nitrite. And I am not even sure how soluble NaNO2 is in alcohol.
[Edited on 29-6-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Quote: Originally posted by AndersHoveland  |
I would suggest trying to react trichlorohexahydrotriazine with sodium nitrite in the absence of water, using propylene carbonate as the solvent. If
there is any water, the nitrite will just be oxidized.
|
Again this, then explain me how are you going to make Cl- from Cl+ (in chloramine, which when hydrolyzed with water gives HOCL, again Cl+ here)
without some sort of redox reaction, I mean no exchange is possible to form AgCL or am I missing something?
[Edited on 30-6-2013 by papaya]
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Sorry to bring up and old thread..
Does anyone have yields on this ?
If the efficiency is high on the conversion of hexamine to trichlorohexahydrotriazine then the reaction with NaNO2 should go to completion forming
R-Salt.
As previously speculated .... I don't see RDX forming.
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Recently I try to synthesize 1,3,5-trichlorohexahydrotriazine without success, using NaDCC instead of hypochlorite. Somebody knows why it doesn't
work?
Without any acid (basic pH in mix, no hypochlorous acid formed) I get a white, non-flammable precipitate. Using acetic acid, just hexamine
decomposition (strong formaldehyde odor).
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
As mentioned above, the electrophilic Cl+ is needed for this reaction. I'm guessing NaDCC is less labile than OCl- when it comes to generating Cl+.
This reaction and product seem useful though. If the product can in fact generate radical species maybe it would be useful as a chlorinating agent. It
would probably give triazine with elimination of HCl.
It's a shame that the NaNO2 displacement doesn't work. Maybe a more typical nitration with H2SO4/HNO3 would work, since the electrophilic NO2+ is
generated. Alternatively, directly gassing a heated solution of the product with NO2 might work, with the release of chlorine gas.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | It would probably give triazine with elimination of HCl.
|
This was the first thing I thought of when I read this thread. Unsubstituted s-triazine is a difficult target; controlled oligomerization of hydrogen
cyanide has been done, but has obvious downsides.
|
|
|
| Pages:
1
2 |
|