| Pages:
1
2
3
4
..
6 |
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
well thats more poisonous than plutonium now isnt it!
|
|
|
paulr1234
Hazard to Self
 
Posts: 51
Registered: 30-8-2010
Member Is Offline
Mood: No Mood
|
|
I think I'd be willing to hold a small piece of Plutonium in my ungloved hand for a second, I don't think I'd do that with a drop of Dimethyl Mercury:
http://www.fortfreedom.org/p22.htm
The former would make a great picture for Facebook !
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by shivas  |
Botulinum is in fact the most acutely toxic substance known. Less than a kilo is enough to kill the whole planet. |
Yes, it is a shame researchers have not yet developed a chemical synthesis for this lethal enzyme.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Such a shame. (sarcasm)
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
1 kilo wow that is truly amazing.. i wonder why no one has weoponised it.. although that might explain some bad takeaway ive had 
|
|
|
HexJam
Harmless

Posts: 25
Registered: 29-9-2008
Member Is Offline
Mood: Steady
|
|
I might be mistaken but I though Batrachotoxin (poison arrow frog venom http://en.wikipedia.org/wiki/Batrachotoxin ) was the most potent neurotoxin... I still wouldn't like to try my chances with diethyl mercury like
hahaha
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
The lethal dose of batrachotoxin is around 100 micrograms, while the lethal dose for botulinum is around 0.1 microgram.
|
|
|
Endo
Hazard to Others
  
Posts: 124
Registered: 5-1-2006
Location: USA
Member Is Offline
Mood: Cold
|
|
It looks like Tert-butyl hydroperoxide rates a 4 in each of the NFPA diamonds, as well as the OX (oxidizer) warning.
Of course we are talking the pure substance and not the typical 70% in water which is much more stable.
http://cameochemicals.noaa.gov/chemical/2692
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
I'm fairy sure (because i remember reading it, lol), that the flavour of peas is the most potent, 3- methoxy-2-isobutyl pyrazine being 'tasteable' in
peas albeit only there in ppb quantities, this opposed to 'smellable' chemicals which are different.
i may remember it incorrectly but hey you do a google search for 'pea molecule' and see what you get, thats another extreme altogether pardon the pun.
i changed it to 'sweetpea' and got a bunch of hits regarding soppy romantic natural ethereal lovey subjects.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by bquirky  | 1 kilo wow that is truly amazing.. i wonder why no one has weoponised it.. although that might explain some bad takeaway ive had  |
I'm sure they have and I'm sure its unworthy, weapons of this nature are hard to make stronger first of all and making a kilo of a substance that
could destroy the world is not on many people to-do list.
Its like the super potent opiates as weapons, they are just to hard to handle to be considered viable weapons. Sure they have been used in Russia
during terrorist ordeals but there is just not much use in super potent weapons other then fear, that's why we have never seen another use of the
Atomic bomb till this day. Total annihilation goes against what one normally looks for in the conclusion of any conflict, we normally look for total
conformity, destroying our opposition is just a sad side effect of oppressing there will power.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
Dicyanoacetylene, highest flame temp achieved by burning an organic compound when it is burned with oxygen. 4990 degrees C when burned in oxygen,
5242 degrees C when burned in ozone.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
how about DNA ? thats an extremely complexe molecule ...the most complexe as far as we know...so far
|
|
|
CZip
Harmless

Posts: 12
Registered: 18-9-2010
Member Is Offline
Mood: No Mood
|
|
What about huge molecules from termosets like bakelite? Product can by made from ONE molecule (for ex. old iron - you know the thing which can your
mom use for making your colthes flat)
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
these are polymeres...they dont count as complexe its the same sequences of atoms...rite?
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
"DNA consists of two long polymers of simple units called nucleotides, with backbones made of sugars and phosphate groups joined by ester bonds. These
two strands run in opposite directions to each other and are therefore anti-parallel. Attached to each sugar is one of four types of molecules called
nucleobases (informally, bases). It is the sequence of these four nucleobases along the backbone that encodes information." -wiki.
A great example neptunium, probably one of the best. This is a great and in depth wiki article on DNA. A great read.
http://en.m.wikipedia.org/wiki/DNA
[Edited on 23-12-2011 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Does graphene count? It's one of the most extreme allotropes of carbon known, with a thermal conductivity exceeding that of diamond and a tensile
strength exceeding that of the carbon nanotube.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
STRONGEST SMELL
T-butyl mercaptan blends which are added to odorless natural gas (cooking uses) and to serve warning of gas leaks.
Note, butanethiol (butyl mercaptan) derivatives are present in skunk secretion.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
There is an interesting article here on Butyl Isocyanide regarding the unfathomable odor. Having smelled both butyl isocyanide and methyl mercaptan I think that the butyl isocyanide
wins the prize but the methyl mercaptan gets points for staying power. Still, we could go on about stench for hours, does anyone know the most
phosphorescent compound by chance?
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Do you mean longest lasting? Or highest brightness under a blacklight? Or both?
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I think he wanted to said the brigthess...
We need to said clearly wath we want to said if we want to be understand.... Personnaly I need some work in english grammar.
[Edited on 25-12-2011 by plante1999]
I never asked for this.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I wasn't being specific because the segue was meant only to put the topic back on track since there is already a whole thread devoted to the worst of
stinks. Phosphorescence just seemed something that is able to be quantified where someone may have known some 'winning' compounds off the top of their
heads.
|
|
|
Alastair
Hazard to Self
 
Posts: 59
Registered: 13-7-2011
Member Is Offline
Mood: Barely any solvent in my emulsion
|
|
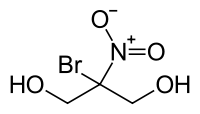
Bronopol
An effective antimicrobial, whose effective use-concentration which can be as low as 0.0025%.
I think its pretty badass, but not incredibly.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Technetium is a fascinating element to me ....with 43 protons its the lowest atomic number with no stable isotope...Tc99 has the longest half life of
4.2e6 y.....baffling!
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
1,5 pentane diamine can be smell at very low concentration if i am not mistaken..cadaverine is its common name for a reason
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Don't forget its equally malodourous cousin putrescine (butanediamine) - sometimes noticed at the very moment it's least expected --- or wanted? 
|
|
|
| Pages:
1
2
3
4
..
6 |