| Pages:
1
2
3
4
5 |
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
I wouldn't use it; mecuric compounds give me the willies! I hope the EPA dosn't hear about the Hg spill its retarted to get so upset over less than a gram of the stuff. its retarted to get so upset over less than a gram of the stuff.
I think if you use a wide mouthed glass next time it will go much faster. Possibly sucking the water vapor/SO<sub>2</sub> fumes off with a
pump would keep them from slipping back into the acid.
We all have our stupid days I remember I destroyed a printer because I got mad at
it. It was a lexmark; shitty printer dosn't like Linux. I remember I destroyed a printer because I got mad at
it. It was a lexmark; shitty printer dosn't like Linux.
What if, what is isn\'t true?
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Theres a small possiblity of refluxing with a narrow necked flask but I wouldnt worry about it. Thunder you got so much H2SO4 I would just pitch what
you have already contaminated with mercury. I have recently lost a thermometer so I feel your pain. However, you need to calm down. Your going to
waste a lot of supplies as a begginer, take it in stride.
Trust me I have wasted a lot of supplies. By the way, you really shouldn't have to monitor temperature when concetrating sulfuric acid.
[Edited on 8-4-2004 by tom haggen]
N/A
|
|
|
thunderfvck
Hazard to Others
  
Posts: 347
Registered: 30-1-2004
Location: noitacoL
Member Is Offline
Mood: No Mood
|
|
I know I shouldn't have to montior temperature, but I was curious. I mean, I'm a beginner, and I have a thermometer, so what do you expect
me to do. BTW, the temperature was 120. haaaaaa. My sulfuric acid is now kind of yellowish in color. I've read that mercurous sulfide
precipitates out of the solution, so any that I may have would precipiate, no?
It's not the fact that it's contaminated, really. OI mean, that pisses me off enough as it is, it's just that I've spent a good
three days concentrating this stuff and for what? A contaminated solution. You probably understand why I blew it.
I swept my basement floor and found no mercury balls. The last time this happened, I dropped a ball of the stuff and it splattered all over ym table.
However, I was able to clean it up once I cleared my table off, I cleaned off many little metallic balls. I didn't find any this time, while I
swept. But these things are SO small, so it's likely that I entirely missed them. How dangerous is this stuff? Assuming I have like mg quantities
scattered on my floor.
The mercury didn't seem to react with the concentrated H2SO4. When the thing broke, it just spilled out and remained a tiny ball. It didn't
bubble or anything. I've read that while preparing mercuous sulfate, you have to reflux it at HIGH temperatures (about the same I had going I
suspect) for quite some time. I had it sitting in there for no more than 3 minutes. I don't know though. When I emptied the flask, there was some
yellow/white crystallized sbtance on the walls of the flask. Things I didn't have thetre before so I accredit it to the mercuous sulfate. Should
I REALLY just get rid of this? I've spent so much time boiling off the water, t'd be incredibly frustrating to just get rid of it all.
And BTW, how do you dispose of it? I know I'm supposed to neutralize it first, but I wasted like half of my sodium hydroxide trying to neutralize
the used battery acid I had retrieved directly from the battery, and it stil wasn't neutralized. I ended up dumpoing it directly into the sewer.
I know that this shouldn't be done, but reagents aren't cheap. That doesn't justify my dumping it however, but, what am I to do.
I'm really not willing to uyse most of my NaOH to neutralize something I'll just end up throwing away. Oh, I'm so evil.
But when that thermometer dropped, I just lost it. There was a brief period where I thought I was okay, then I saw the mercury, and I flipped.
Thermometers are so bloody senstive.
My approxiametly 1 L of H2SO4, from about 3L, gives off what looks like white fumes. However, I believe it is still water that is being driven off
(STILL!). I blow on the vapour, and it turns whiter, presumably because I'm condensing it even more and it turns white because of it. But the
fumes that it's been giving off are not WHITE white, you know? It's just like water vapour. It dissipates quite rapidly and isn't very
irritable to the lungs.
Anyways, I've written quite enough. I'm mainly concerned with the yellow color, I've seen sulfuric acid (concentrating, fuming) that
was yellow before, in my school lab. But I'm not sure about this. As I recall, the solution was not yellow (and this is a faded yellow, more
clear than yellow, but it has the tint) until the mercury was exposed to it.
I guess I should just dump it. But fuck, so much time, such a waste. It's very upsetting.
Right after I do something stupid like this I feel, "you know, if you just wouldn't have fucked with it, you'd be fine, you'd have
your thermometer AND your perfect pure acid" but NOOO, I'm too curious for that and careless. Well, we all learn from our mistakes.
Excuse the length of this, and possible spelling/grammatical errors, I am quite drunk/on MDMA. Soooo,...
Thanks a lot for your input though, you guys rock.
|
|
|
Z-Row
Harmless

Posts: 11
Registered: 23-3-2004
Location: United States
Member Is Offline
Mood: Bemused
|
|
| Quote: |
And BTW, how do you dispose of it? I know I'm supposed to neutralize it first, but I wasted like half of my sodium hydroxide trying to
neutralize the used battery acid I had retrieved directly from the battery, and it stil wasn't neutralized. I ended up dumpoing it directly into
the sewer. I know that this shouldn't be done, but reagents aren't cheap.
|
Why not dump it into the sewer? It is sold as drain cleaner after all, right? You don't have to neutralize it, just dilute it first (and
remember, add acid to water, not water to acid). And there are other cheap bases out there to neutralize acids with, you don't HAVE to use NaOH.
NaHCO3 and Na2CO3 can be found in a grocery store as "baking soda" and "washing soda" respectively (although the sodium carbonate
would be better than the sodium bicarbonate for neutralizing acid) and they are both dirt cheap ( about $.85 US / lb. )
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Mercury is not very toxic in terms of dying, so much as it accumulates in the body, because it is not metabolized and causes brain damage. I
don't think such a small amount will affect you too adversely unless you drink it. I've actually heard a story, I am not sure if it is
true, but in some third world country a poor street performer ritually chugged a liter of mercury and did a belly dance which looked weird because the
heavy mercury in his stomach lagged behind the rest of his body while he danced, and then barfed it back into its vessel. I wonder how long he will
live, if he exists. Also, why do you use mercury, I ordered red alcohol thermometer, they are about $3 each, and when I broke one during a nitro
synthesis, it was no problem.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
silly medeleev, you can't use red alcohol thermometers for high temperature applications. Back in the old days they used to use mercury as a
potion in the non stop search for everlasting life. Ironically these potions brought death. Thunder you have almost motivated me to start a thread
about fail experiments. If you only knew some of experiments that have gone wrong for me...
[Edited on 8-4-2004 by tom haggen]
N/A
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
You can take some sulfur powder and put it on your floor and sweep it around a bit. This should make the sulfide which is relatively inert and lacks
the inhalation hazard in that it does not volitize easily.
|
|
|
thunderfvck
Hazard to Others
  
Posts: 347
Registered: 30-1-2004
Location: noitacoL
Member Is Offline
Mood: No Mood
|
|
That's a nice piece of information, BromicAcid. Although I doubt that I'm in any serious risk, it seems as though most/all of the mercury
fell into my acid. But in the future, if it ever happens again I'll be doing that, seeing as how I DO have sulfur (ah, the excitement!).
As for neutralizing the acid before disposal, using any carbonate will produce that damned CO2. And you could imagine the mess my impatience would
make trying to neutralize all of the acid.
So, should I really waste all of my acid? I could always set this acid aside for showy experiments that I could demonstrate to my friends. But
primarily, at the moment, I want to make some acetic acid/nitric acid. These acids will be distilled, so I'm guessing it would be okay to use an
acid that is contaminated SLIGHTLY?
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
You could bubble in some H2S to precipitate the HgS. The only unknown is how fast/if the concentrated acid will react to just form more HgS and
Mercury Sulfate.
The MSDS says its incompatable with Strong acids. I'm assuming this means it does react. Perhaps rediluting will allow more time to filter out.
This should get more out than with the concentrated. This does of course mean another concentration.
If you're distilling the acids off, I think the mercury contamination should be ok. The mercury should theoretically stay in the distilling
flask.
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
What is the difference between a partial and full immersion thermometer, is it that one can be fully emerged and the other one can't, or is it
that one has to be fully emerged to measure properly, and the other one only a certain amount?
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
ech310n
Harmless

Posts: 29
Registered: 18-10-2003
Location: Australia
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Mendeleev
What is the difference between a partial and full immersion thermometer, is it that one can be fully emerged and the other one can't, or is it
that one has to be fully emerged to measure properly, and the other one only a certain amount? |
I'm sure a Google search would had given the answer you are looking for but here is a link to a page I had in my bookmarks that will explain the
difference: General info on thermometers
|
|
|
thunderfvck
Hazard to Others
  
Posts: 347
Registered: 30-1-2004
Location: noitacoL
Member Is Offline
Mood: No Mood
|
|
Well, I finally did it! My hotplate was out for repairs over the past week or two, and I haven't been able to concentrate my acid. First thing I
did when I got it back was buy a 1 L measuring cup that I felt would make a more efficient container. And so it does! Problem is, I changed stirrers
when I added the acid to this measuring cup, and the solution turned PURPLE. It was very nice but left me slightly unhappy (this was the contaminated
Hg stuff anyway). So I dumped that into my 6L flask and added some activated carbon. A few days later the color's cleared and it looks like this
will be my nitric acid producing batch.
ANYWAY! So I put some fresh acid in the 1 L measuring cup and heated it...Took a few hours to boil down to the point where it started to smoke, but it
smoked. Oh man did it ever. My basement was FILLED with this vile, throat-itching, smoke. It was terrible. Even with the window open...I guess I
really need a fume hood! Anyway, the smoke was enough to set the fire alarm off and that was unpleasant. So now I am currently doing it outside, and
it's quite beautiful really, the thick smokey whiteness that would gag me if given the chance.
Thanks to everyone for their replies!
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
I had a bad run of luck myself today with sulfuric acid. It all started when I walked into NAPA today to replenish my suppy of acid which I had very
recently depleted. Normally they give me the huge 5 gallon containers but today there was a different lady and she gave me a 1 liter container.
That's about 18 times less and it cost me 5 bloody dollars. It you get roughly 200 mL from that that will cost $100 per 4 L of concentrated
acid. At that rate it would be cheaper to buy 4L of concentrated ACS grade acid for $60 from http://www.sciencelab.com and a lot less hassle, thus my frustration. I wasn't about to start a riot over not enough acid so I just paid and
left. Tom Haggen, I believe you said you bought acid at NAPA, do they sell it to you in 5 gallon or 1 quart containers? Maybe they have both and it
just depends on the clerk what you get? Anyway that was the least of the bad luck. I thought maybe I was in luck and this was concentrated 98% acid
that you have to dilute yourself. Not so, I tested it, density=1.245, corresponding to 33% acid. I proceeded to boil it down. The 1 liter took 6
hours to boild down to 98% in a 1L beaker. I take the beaker off the hot plate and proceed to carry it further away from the hot plate for cooling.
When I took the acid off it was at 225C, There was no noticable thumping from uneven boiling but when I took it off, the agitation must have awakened
some feral spirit in the acid for it went "thump". It got onto my hands causing me to tilt the conainer slightly. Enough for another thump
which sent about 100 mL of 225 degree C, >90% sulfuric acid to spill onto my leg. My leg turned black and I now have second degree burns on it and
several enourmous burn blisters. My status as "hazard to self" is most befitting. This has never happened before, usually the thumping is
not nearly as violent. I am not so sorry about the leg as it will heal in time, but I lost a good 200 mL of concentrated acid which stook 6 goddamn
hours to boil down. Any one want pictures of the scars  ? ?
[Edited on 26-5-2004 by Mendeleev]
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
owwieeee.... 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Pics?
POST 'EM!
|
|
|
ShadowStalker
Harmless

Posts: 2
Registered: 3-6-2004
Location: Somewheres
Member Is Offline
Mood: No Mood
|
|
uhh i this this topic is kinda old but anyways
iv noticed with some of(ok most of them) that when its blak is hell if you let it sit for a week or two the lead and trash falles to the bottem and
the acid shude be about 65% pure (hopefully)...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
why make it so hard?
I can understand the problems of the poor folks who live where sulfuric acid is illegal to buy, but why would anyone in the US boil down 35% battery
acid? That seems like a very hazardous and boring activity. After a little internet searching I'm having a 2.5L bottle of ACS grade delivered
to my doorstep. Of course this presumes you have a credit card and don't mind using it.
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Sorry I took a while posting the picture, but anyway, this was taken 48 hours after getting burned. A 2.5 L 98% bottle ACS grade acid costs $70. Up
until recently I've been buying 5 gallons of 35% for 20$ AFter boiling down that leaves about 4 L of 98% pure sulfuric acid. That's 4 L
for $20 or 2.5 L for $70 plus those bastardly hazardous shipping fees. That's $5/L vs $30/L. Hmmmm, which to get, which to get... Besides if
you're going to order everything online why don't you just order whatever it is you're trying to make. The novelty of recreational
chemistry is to able to turn complete crap: yuppy house hold cleaners, batteries, and paint chemicals, into something good: formamide, TNT,
nitroglycerine, meth, etc.
[Edited on 8-6-2004 by Mendeleev]
[Edited on 8-6-2004 by Mendeleev]
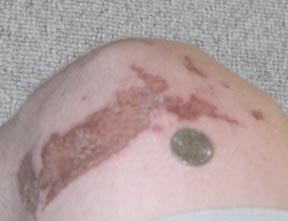
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
Eliteforum
National Hazard
   
Posts: 571
Registered: 18-11-2002
Location: United Kingdom
Member Is Offline
Mood: Enjoying the journey
|
|
Oooch! Looks painful!
If that were mine I couldn't wait for it to scab! Am I alone in liking the picking of scabs?!
All that glitters isn't gold.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
mendeleev, I just bought a five gallon container of H2SO4 33% for like $17. But I've also purchased the 1.5gallon containers. The 1.5 gallon
packages come in a plastic pouch contained in a cardboard box, they are like $7, I scammed them down to $5 one time . My old material science teacher had one of those 1liter containers you bought. But
yes, NAPA carries all three options, sometimes they run out of the big 5 gallon jugs though. You just have to be presistant with those dumbasses. Oh
ya, your fucking crazy for picking up a hot beaker filled with hot sulphuric acid. I always let my glassware cool down on the hot plate. No matter
what the chemical, your damm lucky the glass didn't crack. At high temperatures glassware is very sensitive to temperature change!!! Did it feel
strange when you got burned by the hot H2SO4? The hottest H2SO4 I've ever gotten on my hand was probably 50C and it did some damage to my skin,
yet it was relatively painless. It was bizarre, I don't want it to happen again. . My old material science teacher had one of those 1liter containers you bought. But
yes, NAPA carries all three options, sometimes they run out of the big 5 gallon jugs though. You just have to be presistant with those dumbasses. Oh
ya, your fucking crazy for picking up a hot beaker filled with hot sulphuric acid. I always let my glassware cool down on the hot plate. No matter
what the chemical, your damm lucky the glass didn't crack. At high temperatures glassware is very sensitive to temperature change!!! Did it feel
strange when you got burned by the hot H2SO4? The hottest H2SO4 I've ever gotten on my hand was probably 50C and it did some damage to my skin,
yet it was relatively painless. It was bizarre, I don't want it to happen again.
magpie, unless your a college professor or a licensed pyrotech, I wouldn't be buying chemicals off of the internet.
[Edited on 9-6-2004 by tom haggen]
[Edited on 9-6-2004 by tom haggen]
N/A
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
make vs buy
Mendeleev,
I checked with NAPA before I ordered the ACS grade. I would pay $40 for what I would pay NAPA $20 for in the 5-gallon container. I don't know
what the shipping costs will be but I will let the forum know. Saving the hassle, hazards, and getting ACS grade made it a no-brainer for me. I also
will be getting "Readily Available Chemicals" and purifying as required, but H2SO4 is not one I want to tangle with.
Tom Haggen,
I am neither a college professor, licensed pyrotech, or have a business license. My credo is that I'm not doing anything illegal. Why
shouldn't I buy chemicals over the internet? Sincerely, please let me know.
Magpie
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Pyrex beakers are made to be placed directly on the hot plate or flame, the company says so, so if it cracks while being used in its standard
application and you get injured as a result, you sue for lots of money. Its a completely legit cause to sue if the beaker brakes under 250 C, if the
company says they can be placed directly on a flame. As far as picking it up, I used tongs and gloves, that's why my hands didn't get
killed, just the few splashes on my knee. However I will heed you're advice and leave it on the hotplate to cool next time, because I don't
need something like this to happen to me again  . It never thumps that much, I
don't know what the hell happened. It didn't feel weird when I got it on me, it just felt like any other hot corrosive chemical feels to
the skin, burning a hole in you. That's a pretty awesome deal you're getting magpie, ACS sulfuric acid for $40/2.5 L. I am considering
buying a 750 pound (190 L) drum of technical grade 93% sulfuric acid for $115 from Gallade Chemical, because it is such an awesome deal. It works out
to about 60 cents a liter. I think they sell to individuals. . It never thumps that much, I
don't know what the hell happened. It didn't feel weird when I got it on me, it just felt like any other hot corrosive chemical feels to
the skin, burning a hole in you. That's a pretty awesome deal you're getting magpie, ACS sulfuric acid for $40/2.5 L. I am considering
buying a 750 pound (190 L) drum of technical grade 93% sulfuric acid for $115 from Gallade Chemical, because it is such an awesome deal. It works out
to about 60 cents a liter. I think they sell to individuals.
[Edited on 9-6-2004 by Mendeleev]
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
| Quote: |
Pyrex beakers are made to be placed directly on the hot plate or flame, the company says so, so if it cracks while being used in its standard
application and you get injured as a result, you sue for lots of money. Its a completely legit cause to sue if the beaker brakes under 250 C, if the
company says they can be placed directly on a flame.
|
Of course they can resist 250 deg Celcius, but they will withstand only a small amount of thermal stress. I once placed a pyrex beaker, which had
been, heated on cork to let it cool. It happened that a drop of water was present on this cork and viola, a fine thread streaked through the glass.
Forgot to mention that in my case the beaker of capacity 250cm3, contained only approx 25cm3 of solution in it. I don't know if this small volume
of solution would have affected the cracking, since a small volume cannot 'absorb' as much heat as in the case of larger volumes. In other
words the cooling due to the drop of water occured too rapidly and therefore thermal stress would have occured to a larger extent.
[Edited on 9-6-2004 by Esplosivo]
Theory guides, experiment decides.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Sure there are probably applications where you need to transfer scolding hot chemicals from one container to another. But since my equipment is so
primative I always make a point of letting my glassware cool down on the hot plate. It's not going to go anywhere . .
Magpie, I really don't have a reason for not buying chemicals off of the internet. I just like to keep my name off government lists.
N/A
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Mendeleev,
I have to eat a little crow on my order for the 2.5L bottle of ACS acid. They are not filling my order as they do not ship to individuals.
So I went back to Google and found 2 suppliers who look like they will ship to individuals. You don't know for sure until the order is
completed.
One reason I wanted to bring this issue up for discussion is that I'm concerned about all the forum members who seem to be boiling down sulfuric
acid - it just seems so risky. At one place I used to work a fellow employee was burned badly by room temperature 93% sulfuric acid spraying all over
his leg - very painful, several weeks in the hospital, permanent scars and pain. And he was quite close to a safety shower and used it. I really
hope everyone can find a good supplier (like you have) so they don't have to do this anymore. This is a case where John Ashcroft's
policies are really backfiring on the people he is trying to protect.
Tom Haggen,
I understand how you feel and debated this issue with my self for many months. I finally decided to take a stand that my activities are legal and
proceed accordingly.
This is the other reason I wanted a discussion. I want to know what the concerns and liabilities are with ordering chemicals and equipment with full
documentation, i.e., over the internet with a credit card.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
2
3
4
5 |