| Pages:
1
2
3 |
Oxy
Hazard to Others
  
Posts: 140
Registered: 1-12-2020
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | | I heard moonshining is big in Scandinavian countries, I don't know how they call the process, but the liquor is called snaps. They use to bring their
own snaps to parties as buying alcoholic drinks for many guests becomes expensive real fast. |
I've read somewhere that in Norway(?) it can even bring some attention to buyer. Government agencies can even start procedures which may have severe
consequences like separating from children when they think the alcohol intake is too much.
Norway has however really strange politics when it comes to children. I don't live there but I wonder if it's really such effective as their agencies
want and if it really helps children instead of ruining them psychologically.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
They call it "snaps"?
No doubt that stems from the same root as the german word Schnaps 
Very interesting!
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
Mix of both. Inflation is quite nasty with all the central banks "printing".
|
|
|
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
In Sweden we call homemade ethanol made for drinking "Hembrännt", hem=home, brännt=brewed, distilled.
In my youth i had a stainless distiller rig with 20L capacity.
I made the "Mäsk" with turboyeast + common sugar in 25L pails, it took 4-5 days something to be ready for the distiller.
After distilling, diluting to 40% and purification by activated coal it tastes almost nothing, just a warmth in the chest.
Way better than store bought vodka.
The secret to very good homemade ethanol is the quality of the activated coal.
I still have the stainless distilling rig somewhere but it hasnt been used for 15-20 years.
Maybe i could start using it for making absolute ethanol.
But how to get rid of the last 5% water, molecular sieves?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Good idea to pick up a rig like that! I would start the drying with magnesium sulfate, you can distill off the ethanol as it loses only little water
at those temperatures, or filter.
Better would be CaO though, when you use enough and distill the ethanol you will get anhydrous in one go.
Molecular sieves are nice, but I think they soak up a lot of solvent when used in amounts enough to dry > 0.1% water.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
The 5% water can apparently be removed well with potash, K2CO3.
Although I never tried that.
If it isn't complete, which I doubt it is(but it works great to pull residual water from IPA for example), then you can employ mol sieves.
Distillation over CaO, as Tsjerk also said, is usually the standard method.
Such a large still! 
That would bring customs for tax infringement on the plan here, if they would know... actually, only 500ml's is legally allowed to own without
registration 
But you don't require any registration for labglass, what I told the one or other moonshiner at some point, and a 20l flask from china isn't that
expensive 
|
|
|
Mush
National Hazard
   
Posts: 633
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Schleimsäure  | Quote: Originally posted by karlos³  | Ah the damn new EU laws... yeah, mucic acid, I stumbled upon this new adulterant too 
They are even in the cheaper spiritus brands now... I loved to use the good old AHK spiritus in the clear bottle with the green cap... besides it
getting almost twice as expensive very recently, now they added that crappy IPA to it.
I don't know either.
Just wanted to express my frustration about this new law as well.
PS: I remove MEK usually by refluxing with some NaOH, and then distilling the ethanol off, as the MEK will selfcondense in an aldol to a much higher
boiling compound. |
Ja, didn't know it was new "EU law". Concerning MEK I used once also some NaOH. But hydroxylamine is the safer choice as far as I know, making the
oxime of the MEK. |
Denatured Alcohol Not For Human Consumption EU regulation
| Code: | https://ec.europa.eu/taxation_customs/denatured-alcohol-not-human-consumption_en |
"Per HL of absolute alcohol
1L methyl ethyl ketone (a smelling agent)
1L isopropyl alcohol (a chemical analytical marker)
1gr denatonium benzoate (a tasting agent)
In a few Member States, there are varying combinations of the quantities of two of the three chemical agents, but all three chemical agents are
present in all of the Member States "Euro" formulations for CDA."
EtOH forms a salt alcoholate with Calcium Chloride and Magnesium Chloride .
Attachment: Reaction of calcium chloride and magnesium chloride and their mixed salts with etoh.pdf (770kB)
This file has been downloaded 291 times
[Edited on 18-7-2021 by Mush]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Quote: Originally posted by Mateo_swe  | In Sweden we call homemade ethanol made for drinking "Hembrännt", hem=home, brännt=brewed, distilled.
In my youth i had a stainless distiller rig with 20L capacity.
I made the "Mäsk" with turboyeast + common sugar in 25L pails, it took 4-5 days something to be ready for the distiller.
After distilling, diluting to 40% and purification by activated coal it tastes almost nothing, just a warmth in the chest.
Way better than store bought vodka.
The secret to very good homemade ethanol is the quality of the activated coal.
I still have the stainless distilling rig somewhere but it hasnt been used for 15-20 years.
Maybe i could start using it for making absolute ethanol.
But how to get rid of the last 5% water, molecular sieves? |
I have 50L DIY stainless steel boiler with 1500mm bokakob active reflux column. It can put out about 2 liters of azeotropic ethanol per hour, or
without reflux it can strip at least 5 liters of 60-70%. The column attaches to the boiler with a standard flange and PTFE gasket. It is unsimple to
get very good tasting vodka actually, and I must disagree that store bought vodka would be bad, because all of it is distilled to azeotrope in an
ethanol plant in the first place, and the producers dilute and if necessary, filter it with carbon.
I have used my still a couple of times to strip clean ethanol and toluene: it is easier and a lot cheaper to buy 10 liter canisters of the stuff right
away, and distill them clean at once. The ethanol consists only denaturants and detergents and comes clean over at 78C. Toluene, sold as paint
thinner, contains mostly lighter fractions from 55C, and finally toluene fraction. The cuts are very clean and easy to make with that still.
|
|
|
Keras
National Hazard
   
Posts: 934
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Hoffit  | | I think IPA is a pain to separate from ethanol. I can think of no conventional method that does it easily with typical hobby equipment.
|
Apparently, according to the excerpt attached below from the book Oxidation of Alcohols to Aldehydes and Ketones bleach or molecular chlorine
is able to selectively oxidise secondary alcohols to ketones while primary alcohols are unaffected. Bromine can be used too, so a few drops of a
bromine solution in DCM might do the trick.
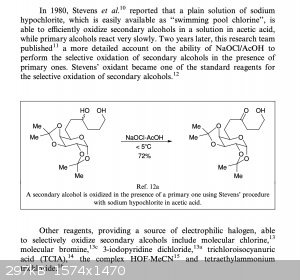
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
According to the paper (and the excerpt) that only works in glacial acetic acid. Bromine and chlorine with ethanol and water give HBr/HCl and ethyl
acetate. Without water you will halogenate the ethanol.
Attachment: stevens1980.pdf (461kB)
This file has been downloaded 266 times
[Edited on 5-8-2021 by Tsjerk]
|
|
|
Keras
National Hazard
   
Posts: 934
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Oops. My bad. But that might suggest another way. Some sort of SN1 substitution which would favour the IPA over ethanol.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Maybe a weak oxidant, secondary alcohols are much easier oxidised to ketones than primary alcohols to aldehydes or even acids?
Always have to think of the oppenauer somehow, but I doubt that is possible here at all, but maybe there are modifications possible to achieve this?
No idea.
I though I'll mention it anways.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Removing isopropanol from ethanol is easiest via a chlorination with hydrochloric acid and zinc chloride.
Isopropanol chlorinates much more easily than ethanol.
But all of the ethyl chloride will distill off easily.
The much greater difference in boiling points will be easier to distill.
Ethyl Chloride (12C)
Isopropyl Chloride (36C)
Isopropyl Alcohol (80C for 91%)
Ethyl Alcohol (78C - azeotrope and pure differ in less than 0.2C)
|
|
|
Keras
National Hazard
   
Posts: 934
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by macckone  | Removing isopropanol from ethanol is easiest via a chlorination with hydrochloric acid and zinc chloride.
Isopropanol chlorinates much more easily than ethanol.
|
So, yeah, that’s basically a SN₁ reaction.
The SN₂ mechanism for ethanol is slower?
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
Quote: Originally posted by macckone  | Removing isopropanol from ethanol is easiest via a chlorination with hydrochloric acid and zinc chloride.
Isopropanol chlorinates much more easily than ethanol.
But all of the ethyl chloride will distill off easily.
The much greater difference in boiling points will be easier to distill.
Ethyl Chloride (12C)
Isopropyl Chloride (36C)
Isopropyl Alcohol (80C for 91%)
Ethyl Alcohol (78C - azeotrope and pure differ in less than 0.2C)
|
Thanks, that is the way to go, I guess. And even economical since HCl and zinc chloride are cheap.
That means for me:
1. First refluxing with hydroxylamin to oximise the MEK.
2. Destilling, getting rid of the oxime and Bitrex.
3. Chlorinate the product with HCl and ZnCl2
4. Destill to separate pure EtOH from 3)
I got a 4l 3-neck, should be worth it.
The hydroxylamine is not the cheapest, but you don't need that much for that bit of MEK.
Thanks again.
[Edited on 5-8-2021 by Schleimsäure]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Keras,
Isopropanol converts via both sn1 and sn2.
With ethanol sn2 is more favorable but the ethanol replacement is less favorable in alcohols and OH is a poor leaving group.
The thermodynamics favor isopropyl chloride over ethyl chloride but not by much.
Both will boil off as they are formed at even modest heat with ethyl chloride boiling at 12C it boils off quickly.
The solution in question is supposed to be 99% ethanol so ultimately more ethyl chloride will likely form than isopropyl chloride even though the
isopropyl chloride is more favorable there is less of it.
You are going to have the same problem with oxidizers as ethanol will oxidize to acetic acid just as isopropanol will oxidize to acetone. Both will
be removed by boiling with a base and distilling.
However aldehydes will oxidize to acids much more readily than alcohols will oxidize with a strong oxidizer.
Regardless of method it is easier to just ferment sugar.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Sugar sufficient for a liter of ethanol is, here in europe, estimatedly around five times as expensive as the cheapest liter of denatured ethanol.
It might be easier, it definitely is actually, but the effort and cost involved makes denaturing denat. EtOH still much more worthwhile.
Good luck I don't need to care about these tiny IPA contaminations 
What an effort to get rid of it!
I'm happy as soon as both MEK and denatonium benzoate are gone, especially the latter, as its much more useful to wash glass thats supposed to be used
for the recrystallisation of something palpatable this way 
Bitrex is literally the worst.
Back when I was still a smoker and washed my glass with denat. EtOH, and then smoked a cigarette a few minutes later, I was hit with all the evil
bitterness that this compound bears with itself from some traces on my fingers 
Horrible stuff!
I always feel like I can taste it again when I'm using the stuff right from the bottle as it is 
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Don't forget drying, hydrochloric is going to add water unless you use gas. Probably still need drying as it is going to pick up water from the air.
|
|
|
Keras
National Hazard
   
Posts: 934
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by macckone  | Keras,
Isopropanol converts via both sn1 and sn2.
With ethanol sn2 is more favorable but the ethanol replacement is less favorable in alcohols and OH is a poor leaving group.
The thermodynamics favor isopropyl chloride over ethyl chloride but not by much.
Both will boil off as they are formed at even modest heat with ethyl chloride boiling at 12C it boils off quickly.
|
Thermodynamics is one thing, but kinetics are another. If the kinetics of the SN₂ is much slower than the SN₁ at the secondary carbon, then you
might be able to get an appreciable amount of isopropyl alcohol out without losing much ethanol.
Thinking of it, thermodynamics won’t help you there because there’s so much more ethanol than the equilibrium hugely favours the formation of the
chloroethane (IMO).
Also, what about KMnO₄. Apparently, permanganate readily oxidises secondary alcohols to ketones, but, unless prodded to, leaves primary alcohols
untouched? Otherwise what says the attached paper.
Attachment: Oxidation_of_2ary_alcohols.pdf (789kB)
This file has been downloaded 255 times
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by Keras  |
Thermodynamics is one thing, but kinetics are another. If the kinetics of the SN₂ is much slower than the SN₁ at the secondary carbon, then you
might be able to get an appreciable amount of isopropyl alcohol out without losing much ethanol.
Thinking of it, thermodynamics won’t help you there because there’s so much more ethanol than the equilibrium hugely favours the formation of the
chloroethane (IMO).
Also, what about KMnO₄. Apparently, permanganate readily oxidises secondary alcohols to ketones, but, unless prodded to, leaves primary alcohols
untouched? Otherwise what says the attached paper. |
My experience is that KMnO4 does not leave primary alcohols untouched. But it does convert them all the way to acid, which is easy to remove. The
kinetics may favor the secondary over the primary.
I know in the chloride reaction the isopropyl is preferentially removed. But short of multiple distillations completely removing the isopropyl
alcohol is going to still leave some residual.
Now one path is convert to everything to the respective chlorides, distill then convert back as the chlorides are much easier to separate. If you
were going for ultrapure, that would probably be the method of choice.
|
|
|
Keras
National Hazard
   
Posts: 934
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by macckone  |
Now one path is convert to everything to the respective chlorides, distill then convert back as the chlorides are much easier to separate. If you
were going for ultrapure, that would probably be the method of choice. |
You're prolly right. But if you have one litre of ethanol to purify, this is going to be a huge task. Using a podgy nucleophile would probably solve
the problem: SN on the isopropyl group would be hindered by steric occupancy, but still possible on ethanol. But we want precisely the opposite… :/
An alternative route would be to esterify with acetic acid, which is relatively inexpensive. Ethyl acetate boils at 77.1 °C whereas isopropyl acetate
boils at 89 °C. They should be pretty easy to separate, provided you use a small Vigreux' column.
|
|
|
Keras
National Hazard
   
Posts: 934
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Wouldn't {urea-hydrogen peroxide} do the trick?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
If you are thinking about esterfication with acetic acid you can just as well go for the selective oxidation with chlorine/bromine. After distillation
residual acetone, ethyl acetate and acetic acid would be removed by refluxing with NaOH.
[Edited on 7-8-2021 by Tsjerk]
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
Quote: Originally posted by macckone  | | Don't forget drying, hydrochloric is going to add water unless you use gas. Probably still need drying as it is going to pick up water from the air.
|
Right, thanks, already thought about that. So maybe dried HCl, over H2SO4 in a gas washing bottle, is better. No need for ZnCl2 then too, I guess.
Otherwise depends how much HCl 37% is needed plus waterfree ZnCl2.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I am sure that oxidation at freezer temperatures, with KMnO4, would preferably pick up the IPA and oxidise this to acetone even at that temperature.
KMnO4 will be more reactive towards primary alcohols like ethanol at increasing temperature....
But, and thats just a slightly educated guess...
A tiny amount of KMnO4(maybe a few times of the amount of IPA contained in there at most) and a night in the freezer, will very likely oxidise almost
all the IPA and almost none of the EtOH.
I know that KMnO4 is still a great oxidant for secondary alcohols, even at freezing temperatures.
But I also know, that primary alcohols aren't that easily and selective oxidised at such cold temperatures.
|
|
|
| Pages:
1
2
3 |