| Pages:
1
2 |
Texium
Administrator
       
Posts: 4606
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
If
you want it to feel less like it’s cheating, why not extract it yourself? In the organic lab that I taught this year, my students did steam
distillation of anise. The NMR spectrum showed that the essential oil obtained appeared to be a ~9:1 mixture of anethole and p-anisaldehyde (no doubt
there were also numerous other minor components present in concentrations too low to register on the NMR). You could take the crude essential oil,
subject it to oxidation conditions to convert all the anethole to anisaldehyde, and then purify the aldehyde by conversion to bisulfite adduct.
|
|
|
Keras
National Hazard
   
Posts: 918
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Texium (zts16)  | | If
you want it to feel less like it’s cheating, why not extract it yourself? In the organic lab that I taught this year, my students did steam
distillation of anise. The NMR spectrum showed that the essential oil obtained appeared to be a ~9:1 mixture of anethole and p-anisaldehyde (no doubt
there were also numerous other minor components present in concentrations too low to register on the NMR). You could take the crude essential oil,
subject it to oxidation conditions to convert all the anethole to anisaldehyde, and then purify the aldehyde by conversion to bisulfite adduct.
|
That’s another interesting experiment, alright. I have a soxhlet extractor that I never used so far, that could be a worthy maiden experiment for
it. TBH I also planned to extract menthol from the tons of mint stalks that grow in my garden.
What solvent did you use?
|
|
|
Texium
Administrator
       
Posts: 4606
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
As I mentioned, it was a steam distillation, so... water. I'm afraid you won't want to bring out the Soxhlet for this one. Steam distillation by
simply mixing the anise with water and distilling will yield plenty of oil, and more can be extracted from the hydrosol with ether, DCM, or
chloroform. If you do a Soxhlet extraction instead, you'll end up with a much less pure extract, since a lot of non-volatile stuff that doesn't come
over in the steam distillation will be extracted too.
|
|
|
Keras
National Hazard
   
Posts: 918
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Oh, great. Sorry, I apologise for not twigging this. It was early in the morning, I was just still half sleepy. Okay, never mind then. I’ll use my
soxhlet for menthol extraction.
Besides I just bought a round bottom flask perfectly fit for steam distillation (see below). I suppose you crush the seed before beginning the
extraction, right?
Thanks a lot!

[Edited on 30-4-2021 by Keras]
|
|
|
Texium
Administrator
       
Posts: 4606
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah, it's best to grind up the aniseed into a fine powder before doing the steam distillation. That type of flask isn't even necessary, assuming
you're wanting to put the anise powder in that flask and pass steam from another flask through it. Honestly, simply putting the anise powder into the
boiling flask with water and running a simple distillation, often referred to as a "wet" steam distillation, as opposed to the more complicated "dry"
steam distillation that you're probably wanting to do, works very well.
I actually just used some p-anisaldehyde yesterday, to make a TLC stain, and to be honest, I didn't think that it smelled all that great. Similar to
benzaldehyde, except with a sharp medicinal odor that kind of overpowered the more pleasant undertones. I think anethole itself smells a lot better!
Nonetheless, it's well worth making to use as a stain, if not for the odor, as it stains different compounds a wide variety of colors, making it
easier to see impurities and verify if you've isolated the compound that you want. See this plate I ran yesterday:
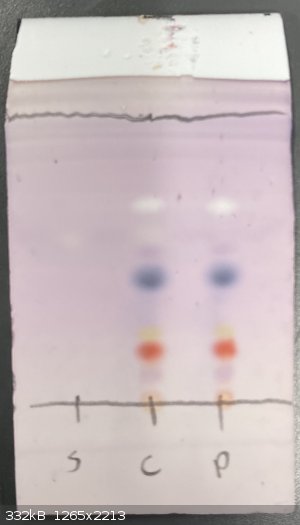
Orange, pink, red, yellow, blue, and white, all from reaction with anisaldehyde!
Edit: the TLC plate pictured is of the crude product from a Suzuki coupling to make a biaryl aldehyde. The desired biaryl aldehyde is the dark blue
spot. Baseline pale orange one is probably triphenylphosphine oxide. No idea about the others!
[Edited on 5-3-2021 by Texium]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Acetic and sulfuric acids are often added. Good/bad?
|
|
|
Keras
National Hazard
   
Posts: 918
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Texium  | I actually just used some p-anisaldehyde yesterday, to make a TLC stain, and to be honest, I didn't think that it smelled all that great. Similar to
benzaldehyde, except with a sharp medicinal odor that kind of overpowered the more pleasant undertones. I think anethole itself smells a lot better!
Nonetheless, it's well worth making to use as a stain, if not for the odor, as it stains different compounds a wide variety of colors, making it
easier to see impurities and verify if you've isolated the compound that you want. See this plate I ran yesterday […]
|
Nice TLC! That reminds me I need to do one to assess the purity of the butyl acetate batch I recently made. Now that is a compound that really smells
good 
|
|
|
Texium
Administrator
       
Posts: 4606
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
The recipe that I used included sulfuric and acetic
acids. My guess is that the acidity in general promotes reactions of the aldehyde: aldols, imine formation, etc. I'm not sure what the reasoning for
including both is, though. One would think that sulfuric acid alone would be sufficient if that was all that was happening.
|
|
|
| Pages:
1
2 |
|