| Pages:
1
2 |
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Don't particularly like4 doing doing business with The Bezos Empire, but cinnamic acid is available on Amazon, and not on ebay *sigh*.
torn between 50g and 500g sizes........
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Synthesis of isopentyl formate: https://www.youtube.com/watch?v=BkQwunQxl8g (my first chemistry video)
Edit: i was informed that i made a safety mistake by using an open flame to distill the ester. I got into that habit because I had a really hard time
distilling esters with boiling points above 200 C, even sand baths cranked up to 10 were not working. and i dislike messing around with vacuum
distillation Next time I do this I would touch the RBF to the hot plate and use an aluminum foil skirt to hold in heat.
it doesn't smell like banana. it's cherry, tart, delicious, reminds me of cherry airheads.
Updated list of esters:
Formate esters
Ethyl formate (ethereal rum)
Isopentyl formate (cherry tart blackcurrant candy)
Acetate esters
methyl acetate (ethereal sweet fruity)
ethyl acetate (ethereal fruity sweet)
propyl acetate (pear)
isopropyl acetate (pear)
butyl acetate (apple)
isobutyl acetate (cherry)
(RS)-sec-butyl acetate (grape)
pentyl acetate (banana)
isopentyl acetate (banana candy)
hexyl acetate (green floral pear)
heptyl acetate (rum woody)
octyl acetate (orange mushroomy)
nonyl acetate (green herbal mushroomy)
Propionate esters
ethyl propionate (pineapple)
isopentyl propionate (real banana fruit)
Butyrate esters
Octyl butyrate (faint waxy pink slightly sweet)
Isobutyrate esters
(none)
Salicylate esters
methyl salicylate (rootbeer minty fresh wintergreen)
isopropyl salicylate (green leather floral)
I am mailing 1 mL samples of esters to anyone who wants to smell them for themselves. Cost is shipping and than 50 cents for each ester. I can also
synthesize a custom ester from any combination of alcohol/acid not yet listed. U2U
[Edited on 20-6-2020 by Cou]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Been lazy and recuperating from traveling, but my esters were waiting for me when I got home.
Isopentyl Propionate--most DEFINITELY banana.
Isobutyl Acetate--smells sorta like ethyl acetate, with a sort of nitrite ester undertone. Not cherries to me.
Octyl Butyrate--waxy indeed. Like the scent of a hard wax candle burning.
Cou, good job. Stuff was packed well, labeled and shipped quickly. I too like stuff that smells good and esters ain't breaking any new ground, but so
what? I too enjoy making them, and by gosh, running a reaction is like working on a car. It CAN be done first go round with a book, but nothing beats
practice to iron out the humps (like violent bumping, they really mean it when it says :"only fill flask MAXIMUM halfway) and org chem is notoriously
finicky and needs practical lab experience to get any good at all. Guess what I am rambling on is that before you can contribute to the field Young
Scientist, ya need some skills and these esters are dang sure doing that, and amateurs with good basic skills have indeed made some pretty hefty
contributions.
Now if I would quit practicing how to make nitrite esters......LOL
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Butyl salicylate:

faint floral blackberry smell
I only got 3 mL, a 24% yield. This was my first time making an ester of salicylic acid and an expensive alcohol, so there were some hiccups that I
learned from.
When making methyl salicylate, you use a large excess of methanol as the solvent, because methanol is cheap and readily available [1]. but larger
alcohols, such as 1-butanol, are specialty chemicals that should be conserved. salicylic acid is very cheap and readily available (I have a 1 kilogram
bag from amazon that cost $35) so it should be used in excess to fully react all the 1-butanol.
Ideally benzene could be used for dean-stark technique, but benzene is expensive (for non-industrial sale), and carcinogenic.
I used a 3 molar excess of salicylic acid to 1-butanol. Xylene was used as the solvent (no dean-stark trap, just reflux). I added too much xylene than
what was needed to dissolve all the salicylic acid. One day maybe I'll determine the solubility of salicylic acid in boiling xylene, but you could add
a little xylene, heat to boiling, then drip it in with an addition funnel until everything is dissolved. Salicylic acid is much more soluble in
boiling xylene than room temp xylene. Less is better
After refluxing for several hours, then allowing to cool, you should gravity filter to remove the unreacted salicylic acid, and wash funnel w/ xylene.
Then proceed to typical fischer esterification workup: water, sodium carbonate, water. A simple distillation removes xylene first, then the salicylate
ester comes out much later (this one boiled at 271 C)
[1] https://www.youtube.com/watch?v=lJLP2bcXDqY
EDIT: I found that salicylic acid is actually more expensive per mole than 1-butanol. It might be better to just use 1-butanol as the solvent. Ideally
you should use benzene as the solvent and use the dean-stark technique, but I'm not sure if I want to mess around with benzene. Once you get to making
salicylate esters of high boiling alcohols, such as 1-pentanol, toluene works as a dean-stark solvent. 1-butanol and 1-propanol are relatively
inexpensive, so you can invest in larger amounts of those to use as excess solvents. I have a huge amount of salicylic acid so I chose to use it in
excess with xylene solvent.
[Edited on 6-28-2020 by Cou]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
When doing fischer esterification, the decision on which reactant to use in excess (or whether you should use equimolar amounts of both with the dean
stark technique) is based on many factors.
Cost: the cheaper one per mole should be used in excess.
Current availability: if you have a lot more of one reactant than the other in your inventory
Ease of extraction in workup: If one is easier to remove from the organic layer in workup, it should be used in excess. Carboxylic acids are easier to
remove because they are either solids that cam be removed by filtration, or they can be ionized with a base and extracted with water. Low MW alcohols
can be extracted easily with water. Long chain alcohols are insoluble in water and must be extracted with a deep eutectic solvent. It's better to use
the long chain alcohol as the limiting reagent.
Boiling points and azeotropes: if both reactants are expensive, the dean stark technique can react an equimolar amount of both to completion. The dean
stark solvent should have a lower boiling point than any reactants or azeottopes. Use "azeotropic data" to search for azeotropes between any 2
compounds in the reaction mixture. Benzene is best for dean stark solvent, but scary and expensive. Toluene has a BP of 110 c. If all of the reactants
boil above 110 C, and there are no low boiling azeottopes, then good to go. This means no propyl or butyl alcohol with toluene. But they're
inexpensive anyway, and easy to extract if used in excess, so things work out conveniently.
The decision is made on a case by case basis. For example, butyl salicylate... if I had benzene I could use the dean stark trap, but I prefer to avoid
benzene. The boiling point of butyl alcohol is too low to be used with toluene. If I had a big jug of butyl alcohol in stock, I would use an equimolar
amount of butyl alcohol and salicylic acid, since they are both about the same price per mole. But at the moment I have about 100 mL of butyl alcohol
and 1 kilogram of salicylic acid. So until i receive large bottles of butyl alcohol and propyl alcohol in the mail, I use salicylic acid in excess
because I have way too much of it.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Cou, 1-butanol drives out a lot of water as it boils as an azeotrope with 55% of water, but very likely it could be some ternary azeotrope due to the
ester present. It separates into 2 layers where upper contains 80% wt. butanol, lower layer water with little of butanol (7,7% wt. butanol). If you
have Dean-Stark trap adapter you could allow the upper layer to return back and drain out only the lower layer so the loses of butanol would be very
small. Yes it is quite cheap so use it in some excess, perhaps 1,5 x more than the acid (calculate the amount according the loses of butanol boiling
with water in azeotrope and add then add some extra amount of the butanol to be sure, it is easy to distill it out. And perhaps no need to use xylene
at all - just my thoughts. Ester with such high b.p. requires vacuum distillation. I expected salicylic acid could be well soluble in the ester, but
apparently it isn't, strange...
Butanol should be well available, there were attempts to use it as a fuel into cars (produced by fermentation process). Here in my country I can buy
from chem supplier analytical grade 1-butanol at a price 12 EUR per 1 liter. So less pure should be even cheaper.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
the BP of butyl salicylate is 271 C. I did a normal simple distillation with heat alone, but any hotter and I would worry about the flask breaking.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Cou, wow you are very brave chemist! I wouldn't distill any chemical with such high b.p. without vacuum... another choice is steam distillation if
there is no risk of hydrolysis...
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Again I forgot to check EVERY azeotrope before doing the dean-stark technique. 1-pentanol and water form a low boiling azeotrope which is great if
you're using 1-pentanol as the solvent, but screws it up when using toluene.
You're right, you can use the alcohol alone as the solvent and water still separates in the dean-stark trap. It's more effective for higher MW
alcohols. I didn't need to be messing around with toluene, but it was worth a try.
if you don't have enough solvent to fill the trap, you can use a 3 neck flask and periodically empty the upper layer of the trap back into the flask.
edit: what source did you use to find that the water layer contains 7.7% butanol? I need a good book of solubilities.
[Edited on 6-29-2020 by Cou]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
https://en.wikipedia.org/wiki/Azeotrope_tables
https://cpb-us-e1.wpmucdn.com/blogs.uoregon.edu/dist/1/8309/...
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
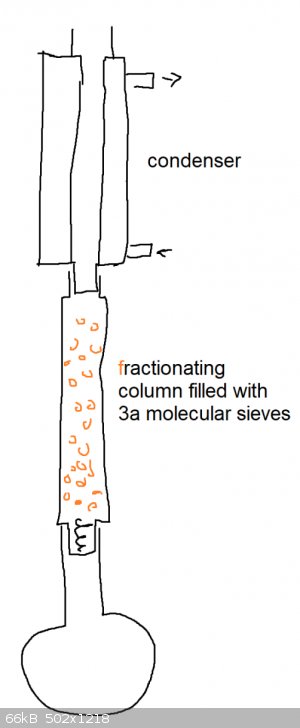
Need help removing water from a small scale (0.06 mole) fischer esterification reaction. anhydrous magnesium sulfate?
it doesn't work with toluene b/c 1-pentanol and water form an azeotrope that screws up separation of water in the trap.
the volume of the reaction mixture is 16 mL
It's too small for a dean-stark apparatus if i don't use a solvent like toluene.
Molecular sieves are not compatible with fischer esterifications.
Can I use anhydrous magnesium sulfate powder to drive fischer esterification to products side?
EDIT
i found that i can use a pressure equalizing addition funnel as a substitute for a soxhlet extractor if i don't have enough volume
[Edited on 7-6-2020 by Cou]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm just going to hitch my wagon to your train here, and report the successful synthesis of methyl pivalate and isoamyl benzoate. Pivalic acid, to
me, smells a lot like gummy candies, and so does the ester- my colleagues thought the acid smelled like shit or rancid socks, but the ester smells
like watermelon or blue raspberry gum. The isoamyl benzoate needs to be purified more before I can smell anything but isoamyl alcohol.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Cou  | Need help removing water from a small scale (0.06 mole) fischer esterification reaction. anhydrous magnesium sulfate?
it doesn't work with toluene b/c 1-pentanol and water form an azeotrope that screws up separation of water in the trap.
[Edited on 7-6-2020 by Cou] |
Magnesium sulfate should be fine, it only loses water at 150 degrees. Maybe you can put it in your PE funnel, it is so course it shouldn't pass the
tap.
Edit: or if you aim for the monohydrate of MgSO4 you only need about 7 grams for a 0.06 mol reaction. The monohydrate only loses water at 200 degrees.
[Edited on 7-7-2020 by Tsjerk]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Very concerned after finding out that coronavirus can destroy your sense of smell.
It can't be completely gone, right? Just means you have to bury your nose deeper in the ester flask. Surely if the air in your nose is a very high
ester concentration, it will still work.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Dallas is damn sure a Covid-19 hot spot right now.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Even in the unlikely case a young person like you would contract that and get serious symptoms like the anosmia you fear, that can still be treated
successful using some cortisone derivatives.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I read that the anosmia is rarely permanent.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
A note on loss of taste and smell.
I was in Italy last May and June when I cam down with a horrible virus infection / flu. Chills, fever, horrible congestion and and terrible cough.
Shortly after I got this I lost ALL sense of taste and smell. Ironically, I was supposed to be attending an intensive food and wine class just after
I became sick. My doctor said that the loss is caused by the virus infecting the nerves for taste and smell and nerve damage can be permanent. These
nerves can be used as pathways for subsequent brain infection. The nerves became infected because they pass near the sinus cavities which were heavily
infected in my case. He said loss of taste and smell is common with serious viral infections. Doc said that recovery of taste and smell could take a
year or more and might never fully recover. After one year taste and smell have still not fully returned.
The flu-like symptoms recurred on my second trip to Italy in September of last year although at a much milder level. This probably affected my
recovery of taste and smell. Interestingly, on both trips my wife did not get visibly sick.
AvB
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
my sense of smell is already very weak to begin with (could be the reason why i like esters so much), maybe it can't get much worse.
|
|
|
| Pages:
1
2 |