| Pages:
1
2 |
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
If you add the urea in small portions and wait until the exotherm has subsided before adding more, runaway should not be possible. The advantage of
heating to 50C is that the reaction is much faster, so you do not have to wait so long between additions and you do not as easily add too much, which
would lead to loss of nitric and could also lead to an uncomfortably high temperature rise.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
What are some legal uses for concentrated nitric acid (not involving energy-rich compounds)? An opportunity has arisen to buy a few dozen liters of
97-98% nitric acid, factory, white, in Teflon canisters. But I can't think of any reason why it would be needed by, say, a small home handyman. All
that comes to mind, refining, jewelry, all of that is solvable and 70% nitric acid. And the big question is, how long would you get to store that kind
of acid? I mean, it's decomposing. I wouldn't be surprised if it's red in a year.
|
|
|
Nemo_Tenetur
Harmless

Posts: 31
Registered: 13-12-2023
Location: Germany
Member Is Offline
|
|
What´s the expected price? Sigma-Aldrich/Merck sell it in Germany for 557 Euro per liter:
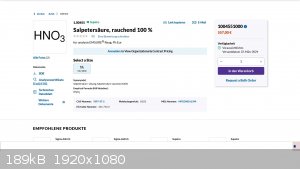
I don´t know in which country you live and about the legal regulations, but to resell it to earn money sounds reasonable.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Well, I'm from Russia (no politics, please, I'm against the war, putin and his oligarchy).
The question is caused by the fact that our turnover of reagents is very bureaucratic, in simple words, what is not prohibited is allowed.
We have so-called precursor tables, there are only three of them. From I with the strictest restrictions, to III with the exception of some control
measures.
For example, acetic anhydride is located in table I, and it is almost impossible to buy it. And sulfuric acid is in Table III, and it is sold upon
presentation of a passport (that you are an adult). The restrictions are mainly aimed at combating the manufacture of drugs, and the sphere of
energy-saturated compounds is almost not affected. Nitric acid is not on these lists.
Therefore, nitric acid of any concentration is sold as NaOH, simply as a caustic substance. And purely legally, it is completely legal.
Another thing is that the sales firms may already have questions. Why do you need it? Is it for yourself? If you show yourself incorrectly, you can
say goodbye to the likely purchase. For example, I was once refused the sale of sulfuric acid. Just because, when asked to whom you are buying, I
accidentally said "to a friend in the garage." That's why I need a reason that could justify buying almost anhydrous nitric acid. So for example, now
when I buy sulfuric acid, I immediately mention that I need it for etching glass together with ammonium fluoride.
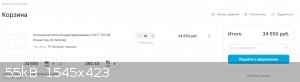
And at the expense of the price, if you take a large container (20l = 30 kg), the cost will be 34.650 ₽ ($ 380.45). This is very expensive (for
many, a monthly salary), while 70% acid will cost $ 60 and 65% will cost only $ 40 (because in Russia 70% nitric acid is produced only as "especially
pure for analysis", and 65% may be technical, hence the difference)
[Edited on 12-2-2024 by DennyDevHE77]
|
|
|
Nemo_Tenetur
Harmless

Posts: 31
Registered: 13-12-2023
Location: Germany
Member Is Offline
|
|
In Europe, we also have schedules for drug precursors since many years and since several years also schedules for explosive precursors. Nitric acid
is since a few years heavy regulated, concentrations of three percent and more is prohibited for the general public. Apart from this, I´m pretty sure
that the so-called three letter agencies watch carefully the trade with explosive and drug precursors even if it is not regulated or prohibited.
So I think the acquisition of such a huge amount fuming nitric acid could draw attention.
If you keep the container cool (if possible, below room temperature) the fuming acid should remain usable for most purposes for a long time. If it
discolors and the concentration decreases, you can distill it under reduced pressure with a dehydrating agent to push the concentration up.
|
|
|
| Pages:
1
2 |
|