| Pages:
1
2
3
4
..
23 |
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Hey Nurdrage
Can my potato chip getting annihilated by Mn2O7 be in the collaborative video effort?
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
i'm sure it can be incorporated in somehow.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by mewrox99  | I would imagine that DMS would be fairly reactive to strong oxidizers.
Here are some you should try KMnO4, Ca(OCl)2, Sodium dichloroisocyanorate |
Though my dimethyl sulfide may have been impure, its reactions are much milder than sulfoxide.
Few drops of the DMS put onto KMnO4 dust just sizzled weakly, no ignition or even visible gas release. The mixture did spray slightly.
Then the DMS dripped onto powdered sodium dichloroisocyanurate caused it to sizzle and gave off white smoke (mixing chlorinating agents and organic
sulfides may be a bad idea!), but again no ignition. Conc. perchloric acid dripped in just gave off slight fumes and turned light beige.
This material was far too odorous to work with, and the unknown impurity made it much worse and was also extremely poisonous. It's the reason I could
not get a good density reading, because I could not bring it in to get a closer look.
I'm done with stinky sulfur compounds. Done.
| Quote: | | As always make sure you do it outside and for the first time use only minuscule amounts to avoid any unexpected surprises |
That's always the sensible thing to do. Even some of these mixtures in the small scale which don't react too vigorously in small amounts, can cause
dangerous explosion in the larger scale. An example is adding KMnO4 to dimethylformamide to form a 20% solution, which has been mentioned to give an
explosion after 5 minutes. Mixing 1g KMnO4 to 5g DMF after some 2 to 5 minutes causes exotherm under gas release, crackling and spraying the
permanganate in all directions.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
I REALLY want to see burning ether, everyone says how flammable it is but I have never seen it.
How about various organometallic compounds reacting with air? Basically doing things with them you aren't supposed to. Maybe grignard reagents can do
this.
Pyrophoric lead and iron.
White phosphorus reacting with air.
Hypergolic reactions with N2O4. PeriodicVideos has some good ones of these
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Burning ether is not that exciting.
It is widely available as an easy start material for internal combustion engines in aerosol cans.
Just spray a little in a tin lid and ignite it with a splint.
It will ignite easily and burn very quickly.
The vapour is a lot heavier than air and will fill containers. The vapour can be easily ignited with a splint on a stick.
Small amounts of ether can be put in a balloon and the balloon inflated. Shaking the balloon will fill it with an ether / air mixture and it can be
ignited with a string fuse.
Butyl lithium catches fire in air and triethyl aluminium burns quite readily. Triethyl borane is flammable and burns with a bright green flame.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Assuming you intend ethyl/sulphuric ether. It burns w/ a colourless
flame like ethyl alcohol. You can put some in the snow and
light it - an interesting effect.
Its flammability is a problem second only to its flash point -45oC
and heaver then air vapour. It processes a tendency to form
explosive peroxides. Storing it in the original tin lined container is
a good idea, however, they are hard to re-cork!
djh
---
I remember back in the
year one... had my tonsils
out. Nurse puts on mask...
It smells terrible.
Blow it away .. sez she. I have
never forgiven her.
[Edited on 30-1-2011 by The WiZard is In]
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
Since most of youtube chemist are in this project,then count me in.
When I play with potassium metal,if I handle it too long with tissue i find it will react with tissue and then spontaneously ignited.
EVIL POTASSIUM!
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Buy tissue, you mean tissue paper right? Not skin?
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
Absolutely,
but honestly,i've slightly burned my finger's skin at the time I don't know it will react like that with tissue paper.
Now I'm more carefull when handling them.
[Edited on 31-1-2011 by kuro96inlaila]
|
|
|
hkparker
National Hazard
   
Posts: 601
Registered: 15-10-2010
Location: California, United States
Member Is Offline
Mood: No Mood
|
|
Yes please be careful 
Back to ideas. White phosphorus could be produced by micro distillation of red P in a glass tube. Once this is exposed to air it will ignight. The
problem I have with this is that it would require a torch to prepare the white P, and if you have a torch well... that kinda defeats the point. Still
an idea though...
My YouTube Channel
"Nothing is too wonderful to be true if it be consistent with the laws of nature." -Michael Faraday
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by garage chemist  | | Other than that, read the chapters about hypergolic rocket propellants in the book "Ignition!", available for download in the Sciencemadness library.
Many hypergolic combinations are listed there. |
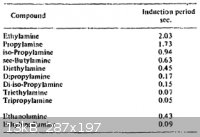
That reminds me. The 4th volume of Urbanski also covers some similar hypergolic mixtures. I've attached a table from Urbanski below, which gives
induction period of WFNA with some organic amines. RFNA or 98% HNO3 and hydrazine or 98% HNO3 and UDMH react faster with induction periods between
0.003 to 0.012 seconds. In the small scale, the hydrazines likely also react more violently.
Quote: Originally posted by hkparker  | | I have mixed calcium hypochlorite and acetone and it doesnt react unless there is water in it to dissolve the ClO-, at least the reaction isnt
visible. I think Ca(ClO)2 will react with many things to start a fire, sulfur sounds worth a try. |
Calcium hypochlorite containing 34% hypochlorite chlorine forms unstable decomposing shock sensitive mixtures with materials like tetralin or mineral
oil according to Gmelin, grind separately if you mix it with anything.
I've never gotten any hypergolic ignitions out of calcium hypochlorite (65% and old, and others) with various substances (brake fluid having glycol
ethers, glycerol, and all else I've mixed it with, the first two which are said to cause ignition).
There is also PbO2 mixed with sugar ignited by conc. H2SO4. I described this reaction here based on crude PbO2. But this is actually more of a glowing than fire. The heat of the reaction might ignite something like a soft tissue.
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
Thanks everyone, keep em coming!
To all the youtubers, and those that want to contribute, start trying some compositions yourself and filming. Don't worry if you overlap, i'll take
the best one or even present them both if i think they're both good.
I'm already trying some of the compositions myself, particularly the more exotic ones requiring lab chemicals.
Quote: Originally posted by hkparker  | Yes please be careful 
Back to ideas. White phosphorus could be produced by micro distillation of red P in a glass tube. Once this is exposed to air it will ignight. The
problem I have with this is that it would require a torch to prepare the white P, and if you have a torch well... that kinda defeats the point. Still
an idea though... |
Its still presentable, basically make a sealed glass tube of the white P with a torch and then carry it around until needed. it could be months later.
Then smash the tube and make fire. sounds great to me.
Afterall on some level making fire using magnifying glass sounds just as self-defeating.... you're using giant stellar nuclear fusion reactor that
outputs more power in a nanosecond than humanity ever will for all time... just to light a fire 
[Edited on 2-2-2011 by NurdRage]
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Give me a few weeks and I'll have ~0,5 kg of white phosphorus to play with. Add to that 1 L carbon disulfide and we're up for some fun. 
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
Quote: Originally posted by Lambda-Eyde  | Give me a few weeks and I'll have ~0,5 kg of white phosphorus to play with. Add to that 1 L carbon disulfide and we're up for some fun.  |
Hmm,dissolved white phosphorus!
Hehehe!
|
|
|
hkparker
National Hazard
   
Posts: 601
Registered: 15-10-2010
Location: California, United States
Member Is Offline
Mood: No Mood
|
|
   1L CS and .5KG of white P! 1L CS and .5KG of white P!
Carbon disulfide has been something I've been chasing for this purpose.
@NurdRage yea I thought that could be a good use, instant fire when you crack the glass! Though you couldn't store it forever, white P will covert
back to red.
I've really wanted to give this a try, but haven't been able to get red P in the US. I have ideas though, let me know what you think. Ill get the
phosphorus from matchbox striker and either distill it into water or dissolve it with CS2 (after I make some). Would it be more practical to just
distill the match head and would water collect the white P well?
My YouTube Channel
"Nothing is too wonderful to be true if it be consistent with the laws of nature." -Michael Faraday
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
That would be suicidal. Slight sloshing of the solution and a film of white P formed might ignite, setting the whole mix ablaze.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Agreed. Thats one of the most stupid, reckless things I have heard in a long time.
If you attempt to try that it will undoubtedly end with the death of you! 1L of CS2 even outside is a serious toxicity risk too
EDIT: Hkparker, I'm pretty sure the only things in the match striker is RP and SiO2
[Edited on 2-2-2011 by mewrox99]
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Calm down, I'm not suicidal or stupid (at least not seriously retarded)! I won't use more than ~10 ml CS<sub>2</sub> with ~100 mg white P
dissolved at a time. And I wouldn't dream of storing the deadly solution!
Also, it would be a terrible waste of such valuable reagents for just one demonstration.
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
good 
|
|
|
hkparker
National Hazard
   
Posts: 601
Registered: 15-10-2010
Location: California, United States
Member Is Offline
Mood: No Mood
|
|
I've seen that solution before, in small quantities, and it seemed stable, I wouldn't scale up though  . Its on mabakken's channel. . Its on mabakken's channel.
My YouTube Channel
"Nothing is too wonderful to be true if it be consistent with the laws of nature." -Michael Faraday
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Hey NurdRage, have you come up with a deadline for submitting contributions? There's a number of things I want to try (especially acetylene + chlorine
for underwater fireworks), but I'd need to order the chems first if we have the time.
With the chems I have now, the ideas I'd like to try are a thermite reaction (with glycerine + KMnO4 to "start the fire"), something with manganese
heptoxide, iodine + aluminum, and the "Negative X" demo that starts fire with water. Any limit to the number of things we can contribute, or should I
just pick the best one?
Looking forward to getting started!
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
currently no limits as to time or how many contributions can be made.
I'll assemble them into a video when i believe i have "enough". like say 15 ways to make fire.
If contributors continue to submit then i made eventually make sequel "15 more ways to make fire". etc.etc.etc.
so go at your own pace, but above all be safe. I'd rather have no videos than no experimenters.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by hkparker  | I've seen that solution before, in small quantities, and it seemed stable, I wouldn't scale up though  . Its on mabakken's channel. . Its on mabakken's channel. |
"Stable" is probably the least fitting description for this solution! It's extremely flammable/pyrophoric as well as being highly toxic, nothing to
take lightly!
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Platinum sponge with fine platinum wire will stairstep into fire with methanol or hydrogen. Also platinum on alumina powder sprinkled into a flask of
methanol will ignite as the minute particles glow falling through the vapor. Do not breathe dust. Fume hood or outdoor experiment.
Calcium Hypochlorite will light with sulflur with time or you can add a drop of water.
No real new ideas though. Seems like there is should be some fun new way to start a fire that hasn't been mentioned, something really exotic.
The fire piston is one of the more unusual ways, very old too.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
This is pretty left field but I wonder if a bombardier beetle could ignite a small vessel with CS2 vapor and air if you directed his abdomen toward
the said arrangement?
CS2 - Auto-ignition temperature: 90°C
Or would his chemical reaction go better in a pure oxygen atmosphere? Seems there must be something he could ignite with his chemical entourage.
http://sps.nus.edu.sg/~yanganqi/angel6.pdf
http://www.youtube.com/watch?v=3sHo8lokQlA&playnext=1&am...
[Edited on 3-2-2011 by Morgan]
|
|
|
| Pages:
1
2
3
4
..
23 |