| Pages:
1
2 |
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Recommend any one trying this is to use a UV-C sterilizing lamp as net output at lower wattage of UV-C will be way larger.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
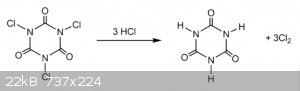
TCCA will dissolve in chloroform. under UV light with aid of little sulfuric acid (or any other acid as catalyst ) they will react to form CCl4.
as shown below HCl will recycle.
i haven't don this before if i did try i will use addition funnel to drip the mixture to my UV chamber which as mono calcium phosphate granules as
solid catalyst. it made from three neck round bottom flask one of which has mercury gas lamp. covered with aluminium foil.
maybe DCM will work too.
|
|
|
Waffle_staffel
Harmless

Posts: 4
Registered: 23-2-2019
Member Is Offline
|
|
For $20, I got a set of 2 HID headlights and removed the outer capsule like Magpie did.
I was inspired by Explosions&Fire's difficulty finding an OTC 320-350nm UV source for his cubane project. I figured I'd just do a proof of concept
which was within my admittedly limited grasp (2 years ago, all a mole was to me was a critter destroying my back lawn).
My overall yield out of 3 runs was probably 40%. The first was, I think, around 70% with one bulb, and the majority of losses were due to overheating
and substantial evaporation. I couldn't really use a hotplate, so I used an alcohol lamp, and I think with the bulbs so close, the heat of the lamps
was more than needed. There was fairly heavy boiling in the flask by the bulbs.
I'm hopefully going to try again this summer, and experiment with different light sources and try a DIY reactor resembling the Vapourtec UV-150, made
out of a spiral of thin walled PTFE tubing.
https://youtu.be/PPy-6LJeUPU
[Edited on 1-14-2023 by Waffle_staffel]
|
|
|
| Pages:
1
2 |