| Pages:
1
2
3
4
5 |
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I LOVE this topic, I envy you guys. I think I might have some picture that could possibly be shared here, I will have a look. But I wanted to share
that I think these samples look absolutely astonishing!
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Nice crystal !!! DrScrabs, super nice. The best gif you can give to someone 
here's some other samples.
   
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Is one of them an aldehyde?
[Edited on 27-3-2019 by monolithic]
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Noup .. They are chromium compounds !!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
A chromate and a dichromate, obviously.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Here are some more compounds 
[Edited on 27-3-2019 by Chem Science]
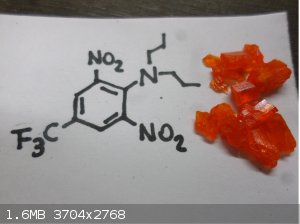
[Edited on 27-3-2019 by Chem Science]
 
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Here are some FLuorine Organic Compounds !! 
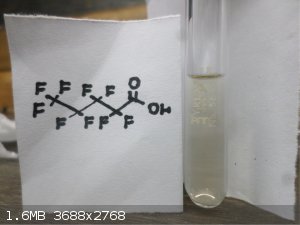 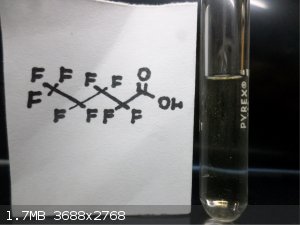 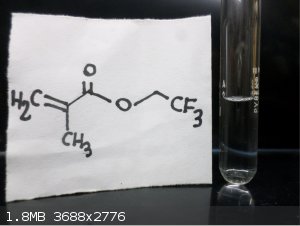  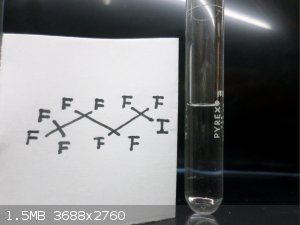
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Where do you get all those compounds? The SCl2 and S2Cl2 can be made by home chemists with some care and effort, but all the other stuff is really
uncommon. You really have access to special chemicals! Very nice!
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Yes the SCl2 and S2Cl2 are easy, the SnCl4 i made it thanks to a SM post <3 The other one are thanks to some years of work in the university, i
want to put them to nice use, so another reason for the post is to see if there is some kind of experiment that will be useful for someone with some
of these i can carry out.. Yea som rare ones but who knows, maybe i can make a fluorinated tertiary alcohol for the sodium project, i dont think it's
needed but it an example.
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|


From left to right, ZnS:Ag, ZnS:Mn, ZnS:Cu phosphors that I synthesized recently.
The ZnS:Ag emits a blue glow, but is so faint that the camera doesn't pick it up. I'm still trying to figure out what's wrong (maybe it's not that
sensitive to 400nm UV light?).
The ZnS:Mn has been made with likely Fe-contaminated MnSO4, and has many dead spots. I'll need to recrystallize the MnSO4 and try again.
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Nice phosphorescent powders beta 4  i had a funny experience with Zn and Cu ..
the first time i made it, it worked .. ten times later i was never able to replicate .... i have to try again. any suggestions ? How did you make
yours ? i made mine with Sulfur, zinc and copper powder ignition. Like i said it worked the first time, but never again i had a funny experience with Zn and Cu ..
the first time i made it, it worked .. ten times later i was never able to replicate .... i have to try again. any suggestions ? How did you make
yours ? i made mine with Sulfur, zinc and copper powder ignition. Like i said it worked the first time, but never again  F*** my luck F*** my luck 
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Fluorescence indeed is very interesting. I also tried making ZnS:Cu from Zn, S and Cu, but the stuff I obtained is very impure. It is a dark brown
solid. Pure ZnS should be white, or pale yellow (??). With some copper included I would expect it to become brown or green (CuS is nearly black, very
dark brown). Maybe I should use very fine copper powder. My copper powder probably is too coarse (65 micron particles). Anyway, the stuff I made does
not show any fluorescence.
Years ago, someone donated me a small display sample of depleted uranyl nitrate (too little to do actual experiments with it, but very nice as
remarkable display sample). This shows fantastic fluorescence under black light.
 
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Nice Pictures Woelen and beta4,
What is your procedure and ratio for making ZnS:Cu?
[Edited on 29-3-2019 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Last year I got quite a bit of Uranium glass (depression glass) some arrived broken so I looked for Uranium salts to "dope" the glass some more.
I felt a bit uneasy at the idea of playing with Uranium salts but now I regret not getting that Uranyl nitrate I was offered !
Great pictures Woelen and Beta4 !
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
I do have some Uranium glass aswell but don´t want to isolate Uranium in any state. Did not find any place wich accepst radiocative waste, also
caontamination of my lab space is unacceptable in that way. Beeing happy with UV glowing glass marbes 
And yes I am very jelous of the compounds posted before!
Edit: writing
[Edited on 29-3-2019 by DrScrabs]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Really cool topic. Chem Science, quite a few of the reagents you've posted have been acids - have you thought of making any salts of them, for
curiosity-sake? Some of them wouldn't be very feasible I suppose (ex. the perfluorocarboxylic acids).
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
If someone is interested in making ZnS:Cu, I posted the procedure and an helpful reference in this thread https://www.sciencemadness.org/whisper/viewthread.php?tid=14....
From my little experience it's key to start from very pure materials. ZnS can be made as described in the Brauer by bubbling H2S in an
acidified ZnSO4 solution, but I decided to just buy lab grade ZnS to avoid messing with H2S.
The copper sulfate was tech grade but I recrystallized it, and the ammonium chloride was made from household reagents but has been recrystallized
three times.
And given this is a thread about reagent pictures, here's a photo of some of my undoped ZnS starting material. I can confirm it's perfectly white.

@woelen: nice fluorescent sample. Does it have an afterglow?
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Here are some nice stuff 
  
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
   
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
 
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
Wow Chem Science, that ruthenium complex is on my list of compounds I want to synthesize, someday. It can be used to make OLED displays.
By the way, the oxalate in your next post looks structurally similar enough to TCPO, maybe it can be used to make a glow stick reaction.
Here are my pictures: triple recrystallized ammonium chloride (white), and copper acetate (dark green).
 
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
yes the TCPO like compound react similar to TCPO .. NileRed has a video about it[https://www.youtube.com/watch?v=Zn61XAGgJA0] I want to make OLED'S
but have not get to that point.
Thar Copper Acetate is Nice !!Ammonium chloride is fairly common, but when it's triple recrystallized ammonium chloride, That gives it Power 
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Sorry, not the best quality of the image. But the PhNHNH2.HCl is triple recrystallized from EtOH and got a nice treat with activated C.
Made by the NaSO3/SO2 reduction(SnCl2 is bs here IMHO), If there is some interest I can post the synthesis next time I make some 

|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@DrScrabs yes!!!
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
MnSO4 made by reacting Mn powder with sulfuric acid. The pure compound should be white, so mine has some impurities.

|
|
|
| Pages:
1
2
3
4
5 |