| Pages:
1
2 |
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by Sauron  | I wish that site wouls shrivel up and die. And if you are their habitue, that explains why you are so intent on benzyl chloride, next step
phemylacetonitrile, after that phenylacetic acid, and then P2P.
Son't expect any assistamce from me. |
I respectfully approach the Dark Lord with a question:
You seem to be vehemently against thinly-disguised drug threads as am I. In this thread you have said you will not offer assistance in the synthesis
of BnCl, which is several steps removed from a precursor to a controlled substance.
But in the Phenyl-2-bromopropane thread, which is currently active, you have offered considerable advice, although this compound is a DIRECT precursor
to a controlled substance.
Do you find these two positions to be somewhat inconsistent, as I do?
I make no accusations, but am just asking about the apparent inconsistency. I'm wondering if established forum members are being granted a "pass"
that is not extended to newbies. If so, I think we should be a little more circumspect about the double standard.
[Edited on 4-7-2009 by entropy51]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I believe sauron is deciding to offer help where he feels it will not be wrongly used. I also do the same.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
May I try and redeem this thread by suggesting a more chemical turn of conversation?
Compared with Xylene, Toluene is boring. Xylene has two methyl groups, and is a mixture of isomers as wall.
Has anyone anything to tell about subjecting Xylene to these oxidation and chlorination reactions?
|
|
|
drfro2
Harmless

Posts: 18
Registered: 1-7-2009
Member Is Offline
Mood: No Mood
|
|
Xylene is still OTC here and readily available,
What reactions can be done with this that are relatively easy?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
"Has anyone anything to tell about subjecting Xylene to these oxidation and chlorination reactions? "
I tryed this very reaction on Xylene to get my feet wet and it makes the run away experianced with toluene seem tame because it takes off very
quickly. Toluene runs away but it does so in a way that you know its comming IE you start to see the Toluene reach a rolling boil and then take off
where as Xylene goes from a few faint bubbles to a hell storm of boiling hot xylene spewing Cl fumes everyware. The hypochlorite route should just not
be used as TCCA is to avalible in a purer form.
Notes say:
| Quote: | | 47.6% Ca(OCl)2 pool shock and Xylene was Heated till temperature reached 105-110 degrees F at which point a runaway takes over and temperature soars
to about 220 degrees F then cools to about 170 degrees in a matter of minutes. leaving with a yellow solution. |
Never tryed to isolate anything because I was just running some test and pretty much playing around at the same time. The bulk of 47% pool shock
prohibited using this in any real sence because 1/4 of the amount thats needed will completely engulf the amount of Xylene or Toluene used.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Did you try portionwise addition over a reasonable period of time?
|
|
|
drfro2
Harmless

Posts: 18
Registered: 1-7-2009
Member Is Offline
Mood: No Mood
|
|
DJF90,
You mention adding portionwise over a period of time,
So if i was to add small portions of Ca(ClO)2 to toluene in the presence of light, spacing additions of the portions by allowing each portion to react
with the toluene, could this lower the chance of a runaway reaction?
Also would having the toluene on an ice bath while adding portions make a difference to this?
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Perhaps you two should read Sedit's advice again, because he has actually performed an experiment:
"The hypochlorite route should just not be used as TCCA is to avalible in a purer form."
Many a big fancy theory has been demolished by one dirty little experiment.
Sedit, thanks for posting your results. I for one am paying attention to your words of wisdom.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
No, more then likely should have though. It was all small test tube scale anyway gearing myself up for the chlorination of toluene. The volume of 47%
Ca(OCl)2 is just to much to perform this synthesis correctly though.
| Quote: | | Also would having the toluene on an ice bath while adding portions make a difference to this? |
Sure would. Reaction can't runaway if its not taking place at all right.
Ill quote from the paper you got your procedure from
| Quote: |
Toluene and dry Calcium Hypochlorite (bleaching powder) are heated together to 105°C in the abscence of other reagents. This avoids by-product
formation. If equal amtounts are used, volume-wise, there is a high conversion. If more bleaching powder is used, the conversion is more robust, but
contaminants such as benzal chloride and benzotrichloride are formed [...] |
Now understand that when this chlorinates HCl is a by product and Ca(OCl)2 + HCl generates more chlorine.... I have tryed a few of the dirty little
experiments as entropy rightly so called them and it was deemed that this reaction just sucks.
It was completely abandoned and the Ca(OCl)2 was used with H2SO4 to generate Cl2 and chlorinated that way instead of this insitu crap. I later found
MnO2 + HCl and heat to be more reliable slow generator as I could speed up or slow down Cl2 generation with a little heat or lack there of.
Here is something I have recently been musing about for the chlorination of toluene. It is known that higher wavelengths work fast but they do not
penetrate the toluene as well so we compromise by using a frequency somewhere around 400nm(sorry cant recall off the top of my head). Has anyone seen
if higher frequency UV feed into the toluene using a bundle of fiber optics as to give larger surface area could speed up the reaction.
[Edited on 6-7-2009 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Sedit  |
Here is something I have recently been musing about for the chlorination of toluene. It is known that higher wavelengths work fast but they do not
penetrate the toluene as well so we compromise by using a frequency somewhere around 400nm(sorry cant recall off the top of my head). Has anyone seen
if higher frequency UV feed into the toluene using a bundle of fiber optics as to give larger surface area could speed up the reaction.
|
Well, allow me to propose this instead: having a quartz tube made like a cold finger with a ground joint without the top portion so that the UV source
can be lowered down into the reaction vessel with minimal absorption by the glass. I've been wondering how much this would cost to have made, since it
is rather custom, but not very complex.
a small air pump may be necessary to help cool the UV source in this case if it's a standard bulb.
However, 1W UV LEDs are available in the range of $10/ea. and put out UV mainly in the upper 300s. I wonder how these compare to typical UV sources.
[Edited on 7-6-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
The source of this prep is apparently US Patent 1280612. It calls for the toluene to be heated to about 90 C and the Ca(OCl)2 slowly added and the
whole mess held at 100 to 105 for an hour with a reflux condenser.
I do not believe that the patent mentions UV irradiation.
I suppose hot toluene might react immediately rather than allowing a buildup of Ca(OCl)2 during an induction period following which it refluxes from
the ceiling.
Danger Will Robinson!
[Edited on 6-7-2009 by entropy51]
[Edited on 7-7-2009 by entropy51]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Addition in portions below the runaway temperature is interesting seeing as it did not APPEAR to react until the runaway occurs at which point the
Toluene turns yellow indicating free Chlorine as opposed to the BnCl. As many know in organic chemistry just because it does not appear to happen does
not mean that its not. Perhaps another small scale adding small spatulas of Ca(OCl)2 is in order to see if it still takes off when the temperature is
raised.
Toluene + Cl2 ==hv==> BnCl + HCl
Ca(ClO)2 + 4 HCl → CaCl2 + 2 H2O + 2 Cl2 <== This is where I feel the runaway is taking place. Its not so much as a runaway chlorination but
a runaway decomposition of the hypochlorite generating heat and speeding itself along at an ever increasing rate.
If the HCl dissolves in H2O is will allow it to react faster then opposed to if it was allowed to slowly boil out at first decreasing the HCl
concentration. At 100 C give or take the H2O formed will start to boil and that could be the cause of alot of problems in the way of a more mobil
reaction. I have not read the patent yet but will do so before attempting any form of test. I would sure like to hear Lens input on this as they have
more knowlage of these reactions then myself.
Also Ca(OCl)2 decomposes under direct sunlight as per,
| Quote: | A diluted aqueous solution of HOCl will decompose very slowly in the dark,
but more rapidly in the presence of light, particularly rapidly in full sun light, by producing hydrogen chloride and
oxygen. Some chlorine and chloric acid (HClO3) may also develop. The physico-chemical properties indicate that
chlorine released into the environment as HClO or Cl2 is distributed into water and air. Consequently, the effects
that may manifest in the natural environment are considered common to those assessed for the other source of
hypochlorite.
In the natural water, in the presence of organic or inorganic compounds, the free available chlorine immediately
reacts forming various chlorinated and/or oxidized by-products e.g. chloramines or chloromethanes. |
Reference:http://www.inchem.org/documents/sids/sids/7778543.pdf
Makes me think that there is a possibility of placing a bottle of Toluene/Ca(OCl)2 out in the sun for a while(days maybe longer) may also achieve the
same product under much more milder conditions. Orrrrrr you could have a massive out gassing of Chlorine and piss off your neighbors and kill all of
your flowers.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Shorter wavelength isn't going to enhance the reaction rate. You need to disassociate Cl2, which means light wavelengths below 488 nm; shorter
wavelengths will leave the Cl radicals with extra energy but only so much is needed to abstract the hydrogen. Past a point shorter wavelengths result
in some of the energy of each photon just being wasted, not an increase in reaction rate. To make the reaction go faster you need more photos in the
target wavelength range.
Look at the reaction rate peaks vs wavelength
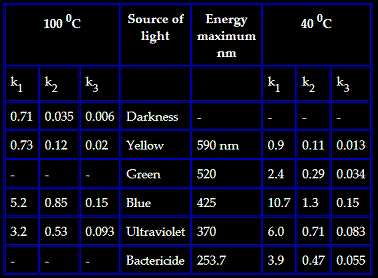
from http://www.sciencemadness.org/talk/viewthread.php?tid=10256
Glass is transparent enough in the wavelength range needed, quartz/silica is just a waste of money.
UV LEDs are also overkill. Ordinary mercury lighting bulbs put out at least 10 times the useful energy per unit cost, as applicable to this
reaction, as UV or even blue LEDs.

|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
I've been thinking of something like that too, UC. Cooling was the first problem that came to my mind, however, a cool stream of air would probably be
adequate.
A standard taper quartz joint isn't that costy, and considering that this apparatus is essentially nothing more than a short, closed tube with a
male joint on the end I don't think it would cost much more than 15-20$ more than the joint itself.
Edit: Typo
[Edited on 7-7-2009 by Lambda-Eyde]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
As I understand it the glass is not what stops the higher wavelenghts but the Toluene itself will not let the UV pass more then a few mm. That web
page that the black chart above is from explains the theory behind the chlorination nicely yet I can't seem to find it again. If anyone knows the
address could you please post it.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
drfro2
Harmless

Posts: 18
Registered: 1-7-2009
Member Is Offline
Mood: No Mood
|
|
I think the site you are talking about is one of these two links,
http://www.faizkaskar.8k.com/light.html
http://www.faizkaskar.8k.com/light1.html
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
That looks like a partial clone of the site I was speaking of but it does appear to have some of the information there. Perhaps my memory just is not
that great.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
| Pages:
1
2 |