| Pages:
1
2
3
4
5 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Arrhenius, I buy reagents whenever possible. Where I live there are a few things restricted, like P. but not I2 and certainly not KI.
I want to make my own MeI to beat the commercial price which is high as you noted and higher here due to shipping and duty. I pay about 2X the
European ex-works prices here, so when something is high ex-works it is very high here.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Ya, I'm not really worried about buying stuff. I just need to expediently discover something new so I can look legitimate  maybe publish some home chemistry in Nature or Science. maybe publish some home chemistry in Nature or Science.
On another note, here's the IR:
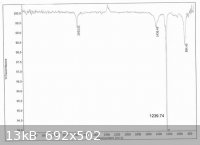
It may be red, but it's the right product. No MeOH in that IR, and it was not re-distilled.
[Edited on 21-6-2009 by Arrhenius]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Turns out that when quenching P4O10 with methanol, 2 mols each of the monoester and the diester are formed from 1 mol anhydride. This consumes 6 mols
MeOH
P4O10 + 6 MeOH -> 2 MeOP(O)(OH)2 + 2 (MeO)2P(O)OH
No water is formed. That equation is balanced, and it is from Ullmann.
Nicodem, will these react with 6 mols KI to give 6 MeI and potassium phosphates?
Is this stoichiometric or are any excesses required?
If no excess needed this could indeed be more economical than using H3PO4.
Sic gorgeamus a los subjectatus nunc.
|
|
|
woelen
Super Administrator
        
Posts: 8060
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
As preparation for the synth of MeI I did some little experiments.
I tried the quenching of P4O10 with methanol. I took 1 ml of methanol and added a spatula full of P4O10. This gives a strong hissing noise and a
turbid liquid with lots of insoluble matter. Not something I expected. I expected a clear liquid and not the slurry I obtained.
Another little experiment I did is dissolving some NaI in methanol (this is amazingly easy, NaI really dissolves well in methanol) and adding this
solution to the other test tube with cooled down methanol/P4O10 mix. No apparent reaction occurs (and I did not expect that). I kept the test tube for
10 minutes or so, and still no apparent change, it remained turbid and white.
Then I took it with me in a sunny place and in the sunshine the liquid turns brown quickly. You really can see the color intensifying from colorless
through shades of golden yellow to brown.
I have to do the distillation outside (due to the carcinogenic nature of MeI) and now I decided to postpone the experiment to an evening during
twilight, when I have the time. Doing it during the daytime with sunlight will lead to very fast decomposition and loss of yield and very impure
product.
I decided to do the experiment with 85% H3PO4, to which some P4O10 is added. I do not want to spend too much P4O10 (which is not at all a common
chemical for me, while H3PO4 can be purchased easily). It is not a matter of financial things, but a matter of availability. For a real lab other
economic rules apply, but for a home chemist there is the additional restriction of availability.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Woelen, we have the stoichiometry from Ullmann that calls for 1 eq P4O10 and 6 of methanol. We do not yet have Nicodem's sage advice as to whether or
not any excess(es) are required, compare the H3PO4 reaction.
Therefore, how do we evaluate 1 mk methanol and "a spatula of" P4O10 to anything? How many g or mmol is a spatula? At least we can calculate how many
mmol 1 ml methanol is. FW 32, d 0.791 so you had 40 mmol methanol and therefore, assuming stoichiometry per Ullmann, you required 6.75 mmol P4O10 FW
285 so 1.92 g.
NaI has FW 150 so the stoichiomeytu calls for 4o mmol of that. But till we know how much that spatula was, we are on terra incognita.
[Edited on 21-6-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
P4O10 + 6 MeOH -> 2 MeOP(O)(OH)2 + 2 (MeO)2P(O)OH
is a very efficient reaction... thanks for taking the time Sauron.
P4O10 is much cheaper than the same amount of oleum or chlorosulfonic acid (P4O10 in quite acceptable grade is something like $25/500g without
shipping, but it's available in 20kg sacks for about that much if one cares to look!). It stands to reason that this method should work--it works with
the acid anhydride SO3 and methanol but with much greater ferocity. When I was working on DMS and DES synthesis, some of the post-reaction mixes were
ink-black from the carbon formed (less of a problem when ether was bubbled through it though).
Sauron, you and I both know it's a trial to get a mass on P4O10 unless one has a dry box or takes elaborate precautions to preclude moisture--how can
we fault Woelen on that?
I expected some of Woelen's results as HI is itself light sensitive (as is MeI) but nonetheless I greatly appreciate hearing about the turbidity of
the solution--perhaps extra methanol may be required as a reaction medium? Also, if this phosphate ester business parallels that of its sulfate
cousins, then perhaps one might actually isolate the ester(s) since at least one of them appears to be a solid (just distill off the MeOH) and then
mix with solid NaI and dry distill! Then again, since there seems to be such an exotherm from P4O10 addition, it would be wise to take advantage of
that when doing a bulk synthesis, and perhaps regulate the reflux of the mixture through addition).
Here is my primary question for Arrhenius: what's in the literature and what isn't? Have you found any papers or work of a similar nature? This seems
like Meat and Potatoes organic chemistry that would've been explored (p)ages ago... if not, a definite contribution can be made: if yields are high,
this would be a good alternative, especially amateur chemists who want methylating agents but don't want the hassle and stigma of using P4 and I2.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
I have tried to attach a literature paper regarding alkyl iodides via the H3PO4 route.
I'm a little suprised that this prep is being discussed as if it's something new. There is quite a bit about it here: https://www.sciencemadness.org/whisper/viewthread.php?tid=16...
Attachment: halides by H3PO4 (Stone).pdf (326kB)
This file has been downloaded 974 times
[Edited on 21-6-2009 by entropy51]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
People always told me that MeI is a horrible carcinogen. This keeps me from trying to make some. Are these rumours true?
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Jor, compared to what? The list of known carcinogens includes Cr(VI), Cd, Ni, benzene, formaldehyde, benzidine, o-toluidine and on and on. The
probable carcinogens are an even longer list. And those are only the ones we know about.
http://www.cancer.org/docroot/PED/content/PED_1_3x_Known_and...
I think any amateur (or professional) chemist who works without adequate ventilation and protective gear such as gloves and sometimes a full face
shield is playing with fire. I treat MeI with a great deal of respect and carefully minimize my exposure to it, but if you have a reliable hood I
don't think you need to fear it anymore than many of the other chemicals you probably use without a second thought.
On the other hand, some of the practices that we've recently seen posted on the forum are certain to result in exposures that will cause health
problems if repeated such that the exposure become chronic.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Jor, MeI is indeed a carcinogen just like many other stuff. This does not mean that people would stop using it, it just means they will not drink or
inhale it. Besides, it depends on your cultural background and education whether you will consider it scary as hell or just a normal reagent to be
treated with some additional care. Within a fume hood I have no problems working with it (though I'm nevertheless glad that my job does not often call
for its use).
Fleaker, there are quite some literature examples about the reaction of alcohols or ethers with H3PO4/iodides and its preparative use for alkyl
iodides, but as far as I remember I never saw any example for the preparation of MeI, just higher alkyl iodides. I never did a thorough search to say
for sure, but it could just be possible that that Arrhenius was the first to apply it to methanol.
(BTW, thanks to Entropy for the interesting paper)
Quote: Originally posted by Sauron  | | Woelen, we have the stoichiometry from Ullmann that calls for 1 eq P4O10 and 6 of methanol. We do not yet have Nicodem's sage advice as to whether or
not any excess(es) are required, compare the H3PO4 reaction. |
I gave my opinion about the optimal stoichiometry a few posts earlier. You probably missed it.
Formally one equivalent of P4O10 activates 6 equivalents of MeOH, however due to the unfavourable pKa2 of H3PO4 the formation of K2HPO4 is
thermodynamically less favoured. Thus 4 activated equivalents of MeOH should react easily (at temperatures of 80°C or less with MeI/MeOH distilling
out), but the remaining 2 equivalents might need a bit more heating. Since the goal is to optimize yields toward NaI which is the price limiting
element, I suggested to use the 4 NaI : 1 P4O10 ratio. I also suggested at least 15 equivalents of MeOH and explained why.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Yes, but I always thought that alkylating agents are a class of compounds with a high carcinogenic potential. It's not very important whether
something is carcinogen or not, it matters how potent it is. And IIRC MeI is very potent, just like hydrazines, dioxins, etc.
But I might be wrong.
I might give it's synthesis a try.
How do you effectively destroy MeI? Is aqeous ammonia sufficient?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Well yes methyl iodide is a potent methylating agent. Although I dont think its quite as bad as dimethyl sulfate. Just use adequte precautions and I
dont see any hassle.
|
|
|
woelen
Super Administrator
        
Posts: 8060
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by Sauron  | [...]
NaI has FW 150 so the stoichiomeytu calls for 4o mmol of that. But till we know how much that spatula was, we are on terra incognita.
|
Please don't give this harsh kind of responses  . What I just did is some testing on
a small test tube scale to get a feeling of what I can expect and how things behave. I always do that kind of tests before I use larger quantities. . What I just did is some testing on
a small test tube scale to get a feeling of what I can expect and how things behave. I always do that kind of tests before I use larger quantities.
There is nothing quantitative in these little tests nor did I claim anything in that reaction. In this particular case, I just wanted to share my
qualitative observations, because they are not what I expected and some of you might also learn something interesting from it (especially the great
sensitivity to light).
If you really want to encourage people to try things then please use another tone! If your tone remains the same like it was in that last post of
yours, then this will be my last post in this thread.
-------------------------------------------------------------------------------
The amount of P4O10 relative to the amount of MeOH used in my little experiment is small. My P4O10 is a free flowing powder and I estimate that the
volume of powder added to the MeOH is less than 1/10 the volume of MeOH. Considering the free flowing properties of the P4O10, quite some air was in
the stuff and then you get an impression how little of this material was added, relative to the MeOH.
I now am setting up things for doing the distillation with 6 grams of NaI (0.04 mol) dissolved in 10 ml of methanol and 3 grams of P4O10 added (just
over 0.01 mol) in another 10 ml of methanol. This makes a fairly large excess of MeOH, but otherwise I cannot get all the stuff dissolved and the
stuff is too much of a slurry. It is getting darker now here, and the time has come to do dark things Sauron - the apprentice of Morgoth, the Lord of
Darkness - likes 
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I am surprised the P4O10 will not dissolve in MeOH.
I will try this too soon, I have about 150g of the stuff. I also have a free flowing powder, but it is extremely hygroscopic, it gets clumpy after
seconds in the air.
If your plain goal is MeI, maybe go the red P route. This will be the route I will take if I ever make some MeI.
And yes Sauron, please be nicer. You are rude to people performing experiments. But we never actually hear a report of an experiment you did. If you
don´t like it the way woelen does an experiment, try it yourself. I´m sure you have all chemicals needed at hand. Besides it would be nice to see a
report with pictures from you performing an experiment 
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Jor, regarding destruction of MeI, strong NH4OH will neutralize residual traces left on glassware. A 1:1 mixture of EtOH and NH4OH is even better IMO.
Finally soak the glass in your base bath of 10% KOH in EtOH.
Obviously don't make more MeI than you need. Its low BP (42.5 C) makes it about as volatile as Br2. Small amounts may be reacted with KOH in EtOH as
described here:
http://books.google.com/books?id=geK5gjEn3hkC&pg=PA269&a...
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Woelen: Interesting results. I've got things set up to do the scale up, but I'm just waiting until I have enough free time.
Entropy: Don't know why, but I've searched the board numerous times and that page has not come up. A few points are brought up in that thread that
are reflected in my prep. e.g. don't need to reflux etc. Honestly, I think people on this board need to be more scientific about their work. If
they were, the "publications" section would contain a lot more work. Agree?
Jor: I would just evaporate methyl iodide outside to get rid of it. I'm not really concerned about the carcinogenicity. Most highly reactive
chemicals are carcinogens (no surprise). Just be careful not to spill a lot, use cold condensors and have good ventilation. I don't have iodine or
red phosphorus, so I won't be taking that route.
|
|
|
woelen
Super Administrator
        
Posts: 8060
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did the experiment but now I already can say that it is a total failure.
I took 10 ml of CH3OH and added 6 grams of NaI (40 mmol). With some stirring this could be dissolved easily and just over 10 ml of clear colorless
liquid was obtained.
I took another 10 ml of CH3OH and added 3 grams of very finely powdered P4O10 in very small portions (just over 10 mmol). Each time when a portion was
added, a very violent reaction occurred with hissing noise. If accidently some larger granules of P4O10 were added to the methanol, then the granule
was covered by a dark brown layer, most likely due to charring of the methanol. After adding all P4O10 the liquid was brown, quite hot and somewhat
opalescent. It was not as turbid as I expected, based on the observations in the test tube experiment. It might be that due to the higher temperature
of the liquid the P4O10 dissolves more easily.
I let the P4O10/methanol mix cool down and then I mixed both liquids. In this way I obtained appr. 25 ml of brown liquid, which is somewhat
opalescent.
This liquid was distilled, using a few boiling stones and a heating mantle for heating. A Liebig cooler was used for cooling and the liquid was
collected in a small 25 ml flask, which was put in a beaker of icecold water. The material was heated very carefully and slowly a colorless liquid
comes over.
Thermometer readout was steady between 63 and 64 C. This is much higher than the boiling point of CH3I. Actually, it is very close to the boiling
point of methanol (64.5 C). Probably my thermometer (which has a range from -10 to + 150 C) is a little bit inaccurate and the main constituent of the
liquid must be methanol 
The liquid I obtained in the collecting flask was perfectly colorless, not any hint of brown could be observed in this liquid. That also is a bad
sign.
Altogether this experiment can be considered a 100% failure. Apparently, P4O10 is not suitable for making CH3I from NaI and CH3OH. I can imagine that
the methylphosphate esters are too stable and that they do not easily give up their methyl-group for exchange with a sodium ion and an electron.
The reaction with acid is Na(+)I(-) + HA + CH3OH --> Na(+)A(-) + H2O + CH3I
The methyl ester reaction would be Na(+)I(-) + CH3A --> CH3I + Na(+)A(-)
Probably the latter reaction does not occur that easily. So, if someone want to make CH3I then Arrhenius' method with H3PO4 is much better than the
method with P4O10.
I did some testing of the colorless liquid I obtained. It is flammable and burns with a pale blue flame, just like methanol, but it is not as easily
ignited as methanol. You have to keep the liquid in a flame for a second or so and then it ignites, while methanol can very easily be lit, just by
keeping a flame nearby a drop of it.
I also added water to the colorless liquid (1 volume of water added to 1 volume of liquid). This results in formation of a white milk-like liquid, not
at all what can be expected with methanol. When more water is added, then the white material also dissolves in the water.
So, I think that I mainly distilled methanol, given the temperature readout of 63 ... 64 C, but that a small amount of another compound distilled with
it as well. This could be some phosphate-based compound, or maybe some traces of a iodide-containing compound?
[Edited on 21-6-09 by woelen]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I would try to use the P4O10 to get to 95% phosphoric acid as in the OrgSyn prep. The distillate temp I collected was from ~50-70ºC. But when water
is added to the distillate, there is clearly a dense layer that separates. Don't chalk it up as a total failure, we all learned something. One thing
you might consider is starting the reaction at 0ºC.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Real interesting woelen. Negative results teach us something. Maybe, with just P2O5 + methanol, the mixture was just not acidic enough. A little
H3PO4 and a reflux might help.
Some time in the future I want to try toluene sulfonic acid as the acid.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Woelen, grow an epidermis, will you? I made no personal attack against you, at all. I merely commented that your report was vague on vital details.
Clearly you have never given a chemical presentation to a university seminar or a chapter meeting of a professional chemical society. My remarks were
not "harsh" or (per Jor) "rude" and I see that they had the intended effect in that you did a much better job with the followup.
My suggestion is that the experiment be repeated with a reflux period prior to distillation. Ude a dewar condenser to preclude loss of MeI
Common sense dictates doing this work in a proper hood or outside. If the latter avoid sunlight.
It is well worth noting that in addition to the H3PO4 method, we have at our disposal the method suggested by entrpy, that is bubbling HCl gas into
methanolic NaI or KI; and that of Fleaker using sodium bisulfate (is Sani-Slush still the brand name?)
I am also confident that the P4O10 method will be proven out as experience indicates that Nicodem is about as infallible as Mycroft Holmes.
Polyphosphoric acid reacts with methanol to form orthophosphoric acid and the monomethyl ester exclusively
H6P4O13 + 3 MeOH -> 3 MeOP(O)(OH)2 + H3PO4
The PPA is prepared by mixing 2 mols of 100% H3PO4 with half a mol of P4O10 (one mol "p2O5") and the 100% acid is prepared by adding the calculated
amount of P4O10 to react with the water in 85% phosphoric acid.
As Nicodem has stated, the monomethyl ester will not react with KI (HI) at the reflux temp of methanol. Might it react with HI formed by adding KI or
NaI to the 3:1 mixture of the ester and H3PO4 above, sans MeOH? Higher temperatures will surely be available! At lest in the 100-120 C range before
etching of borosilicate becomes too serious.
[Edited on 22-6-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Woelen, thanks for your detailed report, I'm sure many members appreciate it. Have you perhaps followed the reaction mixture temperature as well?
Quenching P4O10 without cooling in ice might not have been the best option. The solution turning brown is no good sign.
This week I have a bit less work than usually and was thinking of doing some experiments planed for months (or years), so perhaps I will give a try
this one too.
I did some literature search (sorry about doing this too late, but I can't do this efficiently during the weekends or else I would do it earlier).
There is almost nothing in Beilstein or SciFinder about the nucleophilic substitutions of alkyl phosphates with iodides (this is pretty understandable
since there is nearly no practical use for doing this). Only few examples of which there is a reaction between (EtO)3PO and NaI to give EtI abstracted
from a chinese journal, and there is a synthesis of dibenzyl phosphates by their reaction with iodides (JACS, 77 (1955) 5354-5357, attached).

Maybe, later when I'll have more time I will also compile references for other methods of MeI preparations starting from methanol (but I can already
now say that the only good one is the one starting from NaHSO4 and methanol).
Quote: Originally posted by Sauron  | | I am also confident that the P4O10 method will be proven out as experience indicates that Nicodem is about as infallible as Mycroft Holmes.
|
My experience tells me I'm wrong more often than I'm right. It takes experiments to see what was unexpected, what more needs to be taken into account
and what is to do to make things work. Ideas are cheap, it is experimental work that is hard and tedious, but this also the only thing that gives
scientific results.
| Quote: | | As Nicodem has stated, the monomethyl ester will not react with KI (HI) at the reflux temp of methanol. |
I never stated that. On the contrary, I said that in my opinion all methyl phosphates would react till monodeprotonation is achieved (with NaH2PO4 and
monosodium methyl phosphates being the end products). For dideprotonation (yielding Na2HPO4) more extreme conditions would be needed. That is why I
said the ratio of NaI vs. P4O10 should not exceed 4 : 1. The results presented in the attached paper confirm this. Once the alkyl phosphate
electrophile is deprotonated its electrophilicity drops sharply. In this JACS paper they reacted tribenzyl phosphates so there is no source of protons
to reactivate the electrophile for further reaction, while in a mixture of methyl phosphates resulting from dissolving P4O10 in MeOH there is some.
But still, I would regard these additional two methyls as lost in thermodynamic action, and consider NaH2PO4 as the end product.
Attachment: Dealkylation and debenzylation of triesters of phosphoric acid.pdf (518kB)
This file has been downloaded 940 times
[Edited on 22/6/2009 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Mea culpa. I misunderstood.
So, the 2 mols of monoester are demethylated completely while the 2 mols of diester are demethylated halfway.
In that light, the reacyion of PPA with methanol, which according to Ullnann gives only monomethyl phosphate (3 mols per mol PPA) and H3PO4 may be
worth a try after all, as compared with P4O10 + 6 MeOH only 4 of which can be converted to MeI.
I believe the prep of 1 mol PPA from 85% orthophosphoric, will require less than 1 mol P4O10. Eliminating the 15% water: a mol of 199% H3PO4 weighs
98 g so 15% is 14.5 g water. The stoichiometry of quenching P4O10 with water is
P4O10 + 6 H2O -> 4 H3PO4
So we needn't pull up the calculator to see that less than 1/6th mol P4O10 will be needed to suck up that 14.5 g water. A further 1/2 mol is required
to mix with 2 mols 100% H3PO4 so the total expenditure of anhydride to prepare 1 mol PPA of formula H6P4O13 is 833 mmol.
Something to bear in mind pending more definite resulta from P4O10.
Looking through that old thread from 2004 cited upthread, a remark was made that the literature reported better yields with H3PO4 only when 95% acid
was used along with 2 eq KI. Maddeningly vague and no no refs. However, the source is the Stone & Shechter paper posted upthread. On 1-propanol
as substrate and using 2 eq KI and 2.96 eq 95% H3PO$ and 6 hrs reflux they obtained 90% yield.
Molar ratio alcohol:KI:95% acid 1:2:2.96
[Edited on 22-6-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I thought it would be better if I do this experiment today or else I would just postpone it indefinitely. So this is what happened…
Preparation of methyl iodide from methanol using NaI and P4O10
In a two neck flask (50 ml) equipped with a thermometer, there was dissolved 3.1 g P4O10 (10 mmol) in 12 ml methanol (300 mmol) by adding it in small
portions while stirring intensively on an ice bath. There was lots of hissing when the reagent gets in contact with methanol, but the addition rate
was slow enough to keep the temperature bellow 30°C. To this clear solution was added 5.55 g NaI (35 mmol) and the reaction mixture left stirring at
room temperature for one hour (this was not intentionally, but there were other things to do). The NaI dissolved only slowly and a clear solution was
obtained. A small Vigreux distillation column (1.5 cm wide, 8 cm long) was set on the flask and the reaction mixture heated and stirred on an oil
bath. The first drops of distillate came over at 36.3°C while the reaction mixture temperature was 69°C. A crystalline precipitate begun forming in
the reaction mixture. Up to the reaction temperature of 72°C the distillation rate was relatively rapid (two drops per second with the distillate
coming over bellow 60°C) but then slowly subsided. Meanwhile, more and more solids formed and the reaction mixture became a hard to stir slurry which
caused some minor bumping during the heating. When the reaction mixture reached 100°C the heating was discontinued. When distilling stopped, 10 ml of
water was added to the almost clear distillate (6.03 g) having only a very slight violet tinge. The heavier phase was separated (3.06 g) and dried
with CaCl2 granules which floated on top. This gave 2.8 g of a colourless, very dense oil (56%). The IR spectra recorded on NaCl confirmed the product
identity as methyl iodide:
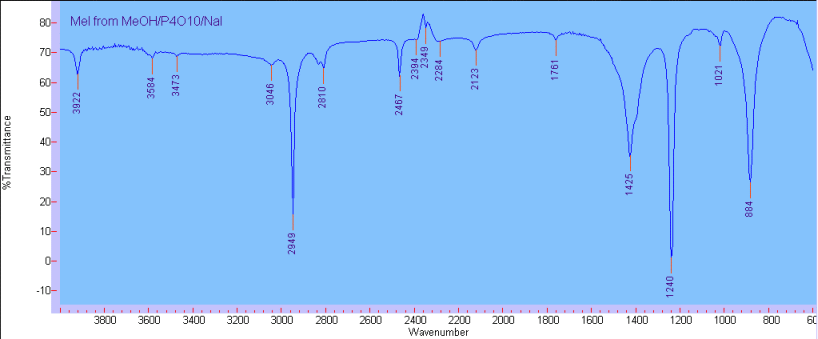
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Let me be first to express my sincere appreciation of your succesful demonstration of this reaction.
Scaling this ti 1 mol P4O10 basis means
310 g P4O10
555 g NaI
1.2 L methanol
c.300 g MeI product
That is acceptable.
[Edited on 22-6-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Fantastic. And when do we get to see pictures?  
|
|
|
| Pages:
1
2
3
4
5 |