| Pages:
1
2 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
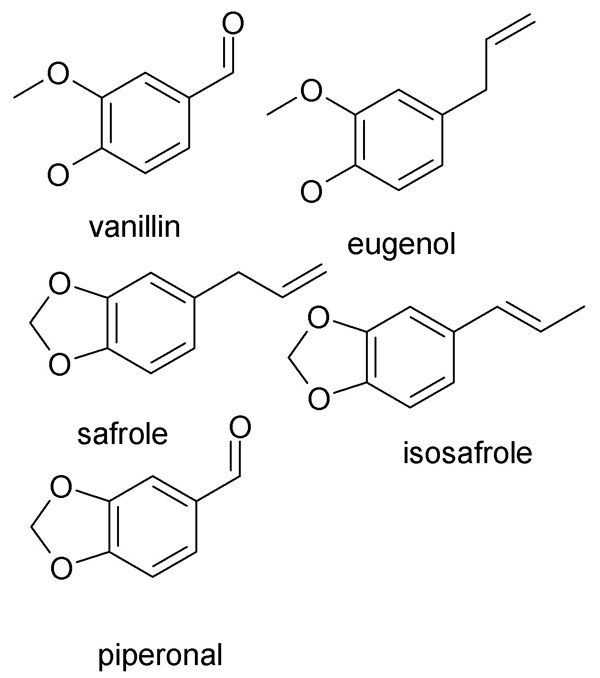
You are right. But safrole, isosafrole and piperonal are listed. I appreciate your posting the link so I could refresh my memory.
Piperonal(dehyde) = 3,4-methylenedioxybenzaldehyde while the safrole/isosafrole system are the isomeric propenyl compounds. As such it is obvious why
DEA wants then under wraps.
Vanillin and eugenol are the 3-methoxy phenols of same series, i.e., vanillin is the aldehyde and eugenol the allyl compound.
ALL of these can be synthesized from phenol to catechol to guiacol and so on. For a speed course (no pun intended!) on why chemrox says all of these
might be listed in the future, just see Rhodium, and its ilk.
Of course I am epistomologically certain that all those threads about these are due to innocent fascination with fragrance and flavor chemistry. Well,
it is a fascinating subject.
[Edited on 19-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
kalacrow
Harmless

Posts: 38
Registered: 23-5-2008
Member Is Offline
Mood: No Mood
|
|
Academic interest, yes  It is frustrating though, that such useful precursors to
so many legal, interesting things are listed. What really gets me is the restriction on Iodine. Man! So many useful compounds are unavailable, and
nowadays you cant easily buy any chemicals as an individual. It is frustrating though, that such useful precursors to
so many legal, interesting things are listed. What really gets me is the restriction on Iodine. Man! So many useful compounds are unavailable, and
nowadays you cant easily buy any chemicals as an individual.
I have my doubts that Vanillin will be listed, as well as Eugenol. They are both traded in HUGE quantities in the food service industries, and are
(were) definitely not anything like as rare in use as safrole and piperonal. Besides, you'd have to be an actual Chemist to turn Vanillin or Eugenol
into the MDxx or other drugs.. I think it would be a very rare cook who could do it. And anyone who is an actual chemist could get there from any one
of numerous routes... you cant list ALL of them, or you'd break whole economies.
Even benzaldehyde is restricted. Eesh. I see its a meth precursor, but it smells so good!!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Oh? Are they traded more than toluene, MEK, acetone, or MIBK? Because they are all listed and you coulkd put the trade in vanillin and eugenol (forget
the others) into a pimple on the left buttock of any one of those.
Does it take a real chemist? If so there are lots of bent real chemists aren't there?
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Many of the listings are meant to target the International Heroin and Cocaine cartels.
The listed chemicals are completely easy to obtain inside the U.S.. I can buy most of them at my local hardware store.
Others listings are aimed specifically at illegal, drug production in the U.S..
Those chemicals may be difficult to obtain here.
To a certain extent, listing is based on threat assessment.
In recent times, those drugs deemed most harmful get the most attention, and the chemicals from which those drugs can most easily be made, get listed.
Coke/Crack....Heroin.....Meth.....PCP....? And then, a bunch of stuff that probably isn't a threat to end civilization as we know it.
Ecstasy and Mescaline are illegal, but making them from Eugenol or Vanillin, isn't exactly easy. And, at this point, Eugenol and Vanillin, are not
listed. If sanity prevails, they will not be. X isn't really that big a problem, and Mescaline is a rare substance.
From some perspectives it appears that the DEA is actually becoming less-reactionary, rather than more-reactionary. Recently, they have been fairly
slow to outlaw materials just because they appear to be somewhat psychedelic. And remarkably, in some cases they have chosen against prohibition.
The Feds have plenty of bigger problems to deal with.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
You are pretty new here, so you may not be aware that the management strongly discourages precisely this sort of discussion. I am not part of The
Management. Just a friendly bit of advice.
I would be really curious as to why KMnO4 and NaMnO4 are all of a sudden listed.
But don't post a reply. PM me or email me.
Sic gorgeamus a los subjectatus nunc.
|
|
|
jarynth
Hazard to Self
 
Posts: 76
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by zed
I'm a bored, over-weight, middle-aged, ex-housewife. |
You don't sound like a woman. You also purport yearlong experience in chemistry-related activities, but your posts reveal your naivete.
| Quote: | Originally posted by zed
From some perspectives it appears that the DEA is actually becoming less-reactionary, rather than more-reactionary. Recently, they have been fairly
slow to outlaw materials just because they appear to be somewhat psychedelic. And remarkably, in some cases they have chosen against prohibition.
|
The DEA has no decisional power on the legislative processes that lead to the regulation of chemicals. It merely enforces the appropriate
policies, although it does have some leeway in choosing which paths to pursue and which to ignore. Due to the mass of information continually gathered
during its operations, it understandably has some influence on the legislators as well.
You seem to have a grasp of drug-related issues in chemistry but apparently don't see the difference in tenor between the various lists of precursors,
or within one list. I highly doubt your local hardware store sells methylenedioxyphenyl derivatives by the pound, or even just acetic anhydride (same
list as acetone).
[Edited on 19-10-2008 by jarynth]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
jarynth, please see my last post above. Polverone does not want drugs-policy debates, period. Not even civil ones. He has closed perfectly couteous
threads containing exchanges just like this. Please, if you want to discuss this with zed, take it private. I would encourage you to delete this last
post and I will do same. I don't want to see this thread locked. I doubt you want to either, but that's where it is headed if you don't cease and
desist.
Sic gorgeamus a los subjectatus nunc.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
KMnO4 b/c of 2-[methylamino]-1-phenylpropan-1-one; I cant remember the trivial name for this.
It is also used in the processing of cocaine.
IIRC some time ago, people were very worried about how easily ephedrines can be oxidized into fairly powerful stimulants.
Bah, mdma from vanillan and eugenol I'll pass! You can probably make piperonal from catechol with higher yields than you can make it from vanillan.
But it doesn't matter, where there's money and guns precursors will always be diverted.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Please do not use this thread, or any other thread really, for broad discussions of drug policy. If you're not here to discuss eugenol, this is the
wrong thread.
PGP Key and corresponding e-mail address
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Sauron, nice images how did you make them? what sw?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
MDL ISIS, then Export as jpg. That actually generates a wmf. Then Photoshop to reduce the image size, and convert to jpg. In the course of that the
image gets nice and bold. Dunno why. It is normal, thin faint lines on screen in ISIS and as a wmf.
Just renaming the wmf as a jpg of course works but as I have to reduce size, better to do both in PS and be done with it.
ISISDraw is free, always has been, though MDL no longer gives away the AutoNom plugin like they used to.
I like this a lot better than ChemDraw, Chemsketch etc.
It also had a plugin to interface directly with AccuModel but now AccuModel is dead and out of business. Oh well. They had an undisclosed 5 yr limit
on my academic license, I guess they don't approve of perpetual students.
[Edited on 21-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I recently purchased a 1 Ounce bottle of "Eugenol, U.S.P." from an online source. The bottle is obviously old, and is from Magnus, Mabee &
Reynard Inc. The bottle contained a dark brown viscous liquid, but it reeks of eugenol. Unfortunately, because the bottle had a linerless cap, it
leaked during shipment. I have two questions.
First, what type of liner should the cap of the new bottle have? Would polypropylene or polyethylene be suitable? (I noticed that the foam packing
materials surrounding the bottle had partially dissolved.)
Second, what should I do to purify this (preferably in high yield)? It should be a clear to faintly yellow viscous liquid, correct? I'm guessing
that what I have is basically an unpurified clove extract. I'm considering steam distillation, but is there a better method or anything I should do
in addition to the distillation? Column chromatography, pelletized Norrit, liquid-liquid extraction (for me, ethers are not an option as solvents)?
I am concerned that following steam distillation, I will be unable to get a high-yield extraction of the eugenol without an ether solvent.
[Edited on 11/6/08 by bfesser]
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
I would think steam distillation. Eugenol is soluble in DCM, do you have that?
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
No, I don't have DCM.
|
|
|
| Pages:
1
2 |