| Pages:
1
..
16
17
18
19
20
..
60 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Possibly but why go to the trouble of making a highly toxic, highly inflammable gas when you can make P from Calgon tablets, some fine sand and some
Al powder?
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
@blogfast25 - depending on where one buys it, Calgon(tm) may contain no phosphorous  - some other products may - one has to look at the fine print on the package. Algal blooms & such are a p.i.t.a.... - some other products may - one has to look at the fine print on the package. Algal blooms & such are a p.i.t.a....
|
|
|
Myfanwy
Hazard to Self
 
Posts: 68
Registered: 20-10-2009
Member Is Offline
Mood: No Mood
|
|
i tested it today with small amounts in a test tube. Even without sand P4 is produced.
you ever smelled the vapours, when white P is stored under water. This odor is really nice and exotic.
Dont know how to describe it better, but its not phosphine.
some strange Phosphorus oxides/acids..
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Mmmmm! I can feel my jaw softening already! 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by densest  | @blogfast25 - depending on where one buys it, Calgon(tm) may contain no phosphorous  - some other products may - one has to look at the fine print on the package. Algal blooms & such are a p.i.t.a.... - some other products may - one has to look at the fine print on the package. Algal blooms & such are a p.i.t.a.... |
If you scroll up, you'll see I made my own metaphospate, as have others here. It can be made from just about any source of orthophosphate...
[Edited on 7-2-2010 by blogfast25]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
In the olden days when matches were made from white P4, the sort that could be struck on any rough surface, "phossy jaw" was common among the factory
workers who were exposed to its vapor. Its symptoms were loss of bone density of the jaws and other soft bones in the head, causing loss of teeth
eventually. Elemental arsenic is at least as bad in this regard, also being a cumulative poison.
|
|
|
Strepta
Harmless

Posts: 44
Registered: 6-5-2004
Member Is Offline
Mood: No Mood
|
|
Attempted Reduction of AlPO4 with Carbon
Introduction
Intrigued by an article posted by SC Wack (Preparation of Elemental Phosphorus), concerning reduction of AlPO4 with carbon @ 1100 C yielding 72% of
available P (IEC vol. 21, no. 11, page 1130) I did an experiment to verify the claim and explore an approach.
Methodology and Fixturing
I used a temperature controlled quartz tube furnace to heat the reactants, fed by a stream of inert or nearly inert gas to sweep the reaction products
toward the exit portion of the tube. This is an improvement of a furnace design I’ve been using for about 5 years which utilizes a ni-chrome element
from a cheap toaster oven re-formed over a ¾” (1.9 cm) threaded steel rod and heated to red-heat to anneal. After cooling, the element is
“screwed off” the mandrel and fitted directly onto a 56 cm section of 18mm OD quartz tubing. The heating element portion of the tube (23 cm in
length and near the input end) is then wrapped with 5 turns of ½” (1.25 cm) kaowool blanket and fitted into a section of 10 cm dia. galvanized
pipe. The quartz tube is cut from a longer section with a dremel diamond dust cutoff wheel and its ends are fire-polished with a MAPP gas-oxygen
torch to anneal the quartz and reduce the chance of cracks. The torch is barely able to fuse the quartz ends. Rubber stoppers are used in each
end—2-hole at the entrance for temp probe and gas inlet tube and single hole at the other for gas exit. A 90 deg tube from this stopper terminates
just under the surface of H2O in a 100 ml beaker. The reaction tube temperatures at each end do not go much beyond 100C when operating at full
temperature. “00” stoppers fixed to the ends of dowel rods will fit snugly into the quartz tube and are used to position and contain the reactant
mixture in the area of the heating coil during assembly. After assembly and final positioning of the furnace, the dowels and stoppers are removed and
the tube ends fitted with the aforementioned 1 and 2 hole stoppers.
The temperature controller is an old design, originally used for an oil bath but now re-biased for control of much higher temperatures. The
temperature probe is a k-type thermocouple (Omega TJ36-CAXL-18G-12) the leads of which terminate at the input to an AD 595. The 595 output is
differenced with a reference voltage and that result input to a SG3524 variable proportional controller which is synchronized to the power line. The
output of the 3524 triggers a triac via an opto-coupler. The triac drives the heating element.
The sweep gas used was “balloon grade” helium stated to be 94-96% He. The tank is a low pressure disposable type pressurized to 18 atmospheres
(260psig) when new. I’m sure these tanks are neither back flushed nor evacuated prior to filling, so I’ll assume they contain 1 atmosphere of air
and 18 of He. This would make the residual O2 content ~ 1%. Because I could not effectively control the flow from the tank using only a needle valve,
I adapted a single stage acetylene regulator to the tank fitting. This involved removing the CGA (Compressed Gas Association) 510 nut and nipple from
the regulator body and replacing with a ¼” flare fitting. All connections to the regulator body are ¼” NPT so commonly available plumbing
hardware was used. The photo shows the tank pressure to be about 40 psig and the output gage pointer is just off the stop at about 2 psig. With the
output needle valve, this arrangement maintains a uniform flow throughout the experiment- set it and forget it. Flow rate used was about 1.5cc/sec.
During a test run, I placed a layer of aluminum oxide (assumed to be inert) throughout the heated length of the tube so that it filled the tube about
halfway (half of tube diameter) and then passed a current of air (aquarium air pump) through the tube. I stepped the heating up incrementally to check
performance. It appeared to work fine up to and including 1250 deg. C was as far as I needed or wanted to go as I was concerned with burning out the
element. Also, although the probe is calibrated to 1335 C, it is not recommended to use it over about 1150 C continuously. The flow rate of air
through the tube was varied to check out the effect on the temperature controller duty cycle. There was not a perceptible change for the modest flow
rates used.
Preparation of AlPO4
I prepared AlPO4 (intended yield 5 g) from 200cc solutions of Al2(SO4)3 (15.5 g) and Na3PO4.12H2O (14g):
Al2(SO4)3 + Na3PO4.12H2O ==> Na2SO4 + AlPO4
When these solutions are mixed, the insoluble AlPO4 immediately precipitates. As I could not be sure of the exact water of hydration in the Al2(SO4)3,
I heated it first to drive off most of the H2O, and for purposes of calculating a weight to use, assumed an anhydrous product. I added an additional
200 ml of H2O to reduce the concentration of the remaining Na2SO4 solution to .1M. The resultant solution pH was highly basic so I incrementally added
Al2(SO4)3 until the pH was lowered to 6.1, the published pH of a .1 M sol. of Na2SO4. The excess liquid was poured off and the remaining liquid and
precipitate were filtered through a Buchner funnel. Yield after drying was 4.8 g out of 5g theoretical.
Reduction
The reaction to be tried was: 2AlPO4 + C ==> 2Al2O3 + CO2 + 2P. The authors used equal weights of AlPO4 and C although this results in a large
excess of C by stoichiometry, possibly to ensure that all of the AlPO4 is reduced.
I added 4.5 g of dried AlPO4 to 4.5 g of carbon black and mixed this in a coffee grinder for two minutes. I was only able to get 4.7 g (of the 9g
total) of this mix into the reaction tube as I wanted it no more than half full. More could have been added by reducing the headspace above the mix
and/or by tightly packing the mix.
4.7 g of reactants would represent .6g available P at 100% yield. With a yield of 70% this amount would be reduced to about .4g.
Experimental
When finally assembled and ready, I started the gas flow and let it run about 10 minutes before ramping the temperature to 650 C. After allowing the
initial transient to settle, I continued to ramp the temperature in 100 deg increments every 5 minutes. As the temp transitioned from 1050 to 1150 C,
the exit portion of the tube darkened and the exhaust gas bubbles burst into flame as they surfaced in the beaker. I continued the temp ramp to 1250
C. After a few minutes at this temp, the probe readings (monitored at the 595 output) became erratic, dropping to as low as 600 and to as high as
1400. I suspected a poor connection in the circuitry or a failure of the 595. After a few minutes, I shut the power off to the controller but left the
gas flowing and allowed the tube to cool. As it cooled the readings became steady again and at 250 C I removed the insulation and quartz tube from the
galvanized pipe section and unwrapped the kaowool. As I unwrapped the tube it broke into two pieces. (Fig 7) The area under the heating element was
extensively cracked, and through handling, another section broke off. I removed the tc probe and found that the Ni-Chrome sheathing had melted and the
melt had largely gathered into three globules. One of these had a vitreous solid adhering to it –this appeared to be a piece of melted quartz.
Some phosphorus was evident in the exit section of the tube (Fig 9) but this was not recovered. About 2.7 g of the original 4.7 g of reactants was
recovered (Fig 8) and this had not fused but was still a loose powder as described in the article.
Conclusions
It appears that AlPO4 is reduced by carbon in the vicinity of 1100-1150 C as described, although I was not abIe to confirm the % released (claimed as
72-83% after 1 hr) as the apparatus itself was also reduced to junk. It also appears that a carbon reaction, presumably with O2, is responsible for
the extreme temperature excursion experienced. I had not thought of it at the time, but the He tank I used had been sitting in the garage for over a
year since it’s purchase. In that time the pressure had dropped from 255 psig to 50 psig. If that represented He preferentially escaping vs air, the
O2 content could have re-enriched by a factor of 5 to ~ 5%. That could have been enough for a low-level charcoal fire to have taken place in the tube.
I will probably try this again after I have replaced the quartz tube, perhaps with CO2 as the sweep gas. CO2 will nearly certainly be reduced to CO at
temp in the excess carbon environment and suitable precautions must be taken to ensure safety. Also, this gives me an idea for a very hot tube
furnace, with temperature modulated by air flow. Materials (alumina tubes, type R thermocouple) would be expensive.

Fig. 1 - Reaction loaded and captured under heating element

Fig. 2 - Assembled, ready to begin
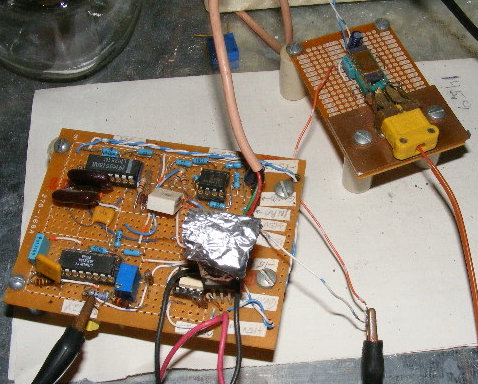
Fig. 3 - Temperature controller

Fig. 4 - He tank and gas regulator

Fig. 5 - Inlet end of furnace at temperature

Fig. 6 Exit end of furnace and gas beaker at temperature

Fig. 7 - Broken tube, temp probe and residual reactants

Fig. 8 - Carbon glow

Fig. 9 - Some P condensed in exit section of tube
[Edited on 28-2-2010 by Strepta]
[Edited on 28-2-2010 by Strepta]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Strepta, I think this is very nice work for a first attempt, especially if that is really P condensed in the exit section.
Might it perhaps be better to embed or otherwise surround the heating element with some type of refractory that would give a more uniform temperature
distribution in the quartz and avoid hot spots from direct contact with the hot wire? I guess refractories would have low thermal conductivities and
might not work as I am thinking.
Better luck on your next try, which I hope is soon!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very interesting experiment. I'm intrigued by the gas bursting in flame after passing through water in the beaker. Surely this is CO, right? I
never saw this with my ZnO reduction with carbon.
I suggest you look into buying an argon cylinder: not too expensive, refillable, and readily available at your compressed gas dealer.
I agree with entropy that it would be better to wrap your heating element around a ceramic tube such as one made of Al2O3 or mullite. I presume you
have seen the designs of garage chemist and myself. I have taken my Al2O3 tube up to 1300C many times with no apparent problems.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The spontaneously flammable gas may have been phosphine but that would beg the question - where did it get the hydrogen?
The breaking element could have produced a hot arc which would melt quartz and possibly account for the fluctuating temperature.
I'm guessing though!
But you sure came close - next time (or the one after that?) could be entirely successful if you decide to go through it one more time.
|
|
|
Polverone
Now celebrating 21 years of madness
|
Threads Merged
28-2-2010 at 16:05 |
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
There could be moisture in the tank, but not very much. The charcoal could adsorb a lot, though. What source of charcoal, was it calcined before
use?
Tim
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Strepta
"The sweep gas used was “balloon grade” helium stated to be 94-96% He. The tank is a low pressure disposable type pressurized to 18 atmospheres
(260psig) when new. I’m sure these tanks are neither back flushed nor evacuated prior to filling, so I’ll assume they contain 1 atmosphere of air
and 18 of He. This would make the residual O2 content ~ 1%"
I think you have much more O2 than you realize. To keep children enticed by the high pitched voice from suffocating if they inhale the gas every
balloon grade He I have seen contains much oxygen. I ran into this trying to build a lifting device where I was experimenting with carrying a wire
high into the air in an experiment measuring the voltage on the wire. To save money I tried balloon gas from more than one vendor eventually having to
buy a tank of pure He. The amount of oxygen was so high it was negating the lift I needed without resorting to filling an unrealistic number of
surplus weather balloons joined together. Meaning of course the entire 5 or 6 percent is oxygen not the 1 percent you think it is.
[Edited on 3-1-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | I suggest you look into buying an argon cylinder: not too expensive, refillable, and readily available at your compressed gas dealer.
I agree with entropy that it would be better to wrap your heating element around a ceramic tube such as one made of Al2O3 or mullite. I presume you
have seen the designs of garage chemist and myself. I have taken my Al2O3 tube up to 1300C many times with no apparent problems.
|
I'll second both of these suggestions. Argon is a good choice of carrier gas. It's cheaper that helium and
doesn't leak out of every nanoscopic pore.
The reason to use a ceramic tube is to mechanically support the heating element. The strength of heating wire goes down at high temperature,
eventually enough that it can't support itself. My guess is that you had a hot spot on your reaction tube that made a weak spot in the wire. That in
itself wouldn't have been a problem, unless the wire were subject to mechanical forces. As a wire loosely wrapped around a tube, it was free to move.
The hot spot would have caused differential thermal expansion at that point, which is enough to generate such forces. By cementing heating wire to a
fixed ceramic tube, it constrains the motion of the wire, greatly reducing the need to rely on the mechanical strength of the wire itself.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Balloon grade helium is 94% He and the rest being O2 to stop suffocation of poeple who breathe it soley to get a high voice :O
Maybe use N2 carrier gas? Cheap and more effective!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Edit: deleted double post.
[Edited on 1-3-2010 by Picric-A]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Frankly, He is too rare (on Earth) and valuable to be used just for party balloons and unmanned advertizing blimps (for which H2 could be used), or
for inert-gas applications for which the much more common Ar can be used. Because the only economic source of it on Earth is from certain deposits (as
in Texas) of natural gas contained in sedimentary rocks derived from granite, which contains U-238 and U-235 and K-40, the alpha-decay of which (and
of daughter isotopes) gives rise to He nuclei, its availability (by separation from these natural gas deposits which are likely to be exhausted by
2100 at the present rate of consumption) is quite finite. While there is a small amount of it in the atmosphere, it is too small for economic
extraction by fractionation of liquid air; and once in the atmosphere it is slowly lost by diffusion (like gaseous H2 unless oxidized) into space due
to its low atomic weight.
|
|
|
Strepta
Harmless

Posts: 44
Registered: 6-5-2004
Member Is Offline
Mood: No Mood
|
|
I should clarify my objectives a bit. In the above experiment with AlPO4 I wanted to verify that P was reduced with carbon at or near 1100 C in the
yields described (72%) and stimulate some discussion of this as an approach. My longer term interest is in exploring paths for the preparation of
elemental P at the amateur level. This assumes no extreme temperature capability such as carbon resistance tubes, MoSi2 heating elements, arc
furnaces, etc, but limited to the temperatures attainable in resistance wire tube furnaces such as those nicely documented by garage_chemist and
Magpie or other readily available means. The chemistries exlored to date include:
1) Reduction of Pb3(PO4)2 with H2 @ 700 C
2) Reduction of NaPO3 with pyro Al
3) Reduction of AlPO4 with C @ 1100 C
I've also conducted some experiments atempting to reduce H3PO4 by adsorbing it onto activated charcoal and heating in a microwave but had no success
with that approach.
@ Magpie: The flames may well be hot CO oxidizing to CO2 as it emerges from the water. I do not recall seeing any "smoke".
As far as the constuction of the apparatus goes, I've used this before to 1200 C without incident. I don't believe it was an issue with the
resistance wire on the quartz (which did not burn out by the way) so much as a "charcoal fire" in the tube. The inconel sheathing of the thermocouple
probe melted off implying temps on the order of 1400 C. The vitreous deposit on one nodule of sheathing would likely have been from either the quartz
tube or the AlPO4 --either would imply temps > 1500 C.
@12AX7: I preheated the C to 450C but it was not truly "calcined" and did contain some moisture as was evidenced in the exit portion of the tube when
first heated to 650 C. I allowed this to dry out with the sweep gas before further ramping the temperature.
[Edited on 2-3-2010 by Strepta]
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
@strepa - Your careful description of your experiment is a model of clarity - congratulations!
If you get Ar from a welding supply house (usually by far the least expensive place to get it!) be sure to ask for pure Ar since welding Ar usually
has 1% O2 in it because it improves the weld quality.
For such low pressures and low rates of flow from a high pressure (140 bar/2000 PSI or so), a "two stage" regulator would perhaps be beneficial. They
are quite expensive new but can be had for $40 or so on EBay or other surplus sources.
Nichrome heating element wire is surprisingly(?) inexpensive from companies who deal with it in quantity. Arklay S. Richards www.asrichards.com sells standard thermocouples and bulk resistance wire at reasonable prices. I had them fabricate 25 simple type K thermocouples
for me at about $1 each - 100 wouldn't have cost much more. They sell bulk nichrome and if I remember their web site, they will wind it into heating
element form for a not excessive cost. I don't know if they sell Kanthal or other super high temperature elements.
Mullite/Al2O3 tubes show up as surplus or overstock occasionally at industrial or scientific suppliers - I got two 1" x 36" x 3/16" wall mullite tubes
for $12 each a while back.
I -think- the AD595 has a "broken thermocouple" indication on some output. I've always bought complete temperature controllers. The Omron 1/16 DIN
size (universal temp sensor input, relay outputs for 1A or so) used to go for $40 or $50 on EBay. They come calibrated and have a lot of features like
proportional-integral-differential control which make operating the furnace more consistent.
Perhaps some of the furnace details should be archived for reference?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
@strepa: In looking at your pictures again there appears to be a considerable amount of yellow flame within the quartz tube. GC says that P burns
with a characteristic yellow flame. Perhaps that is P burning. But I'm not sure where the O2 would be coming from other than the He tank or a leak
of some kind. If it was just a small amount of O2 coming in with the He you would think that your large excess of carbon would take that up as CO.
[Edited on 2-3-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by Magpie  | @strepa: But I'm not sure where the O2 would be coming from other than the He tank or a leak of some kind.
[Edited on 2-3-2010 by Magpie] |
Commercial He used for filling balloons is not pure He but is cut with air which is the source if thats where he got his He from.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Strepta
Harmless

Posts: 44
Registered: 6-5-2004
Member Is Offline
Mood: No Mood
|
|
Sorry about that last post (removed). I'll edit the photos down in size and re-post later.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Not quite sure why you didn't go with the NaPO3/Al/SiO2 reaction. With this very interesting set up (but using Argon welding gas instead of He), you
should have had some nice phosphorus by now.
At those temperatures, consider also the original method: bonemeal (calcium orthophosphate), sand and carbon...
|
|
|
Strepta
Harmless

Posts: 44
Registered: 6-5-2004
Member Is Offline
Mood: No Mood
|
|
I took some photos of the quartz tube from my AlPO4 experiment near the point where the tube broke. There is a white, porcelain-like circumferential
deposit inside the tube which is fuzed to the quartz wall. Everywhere this deposit is present, the quartz is shattered. It's likely that the tube
failure was due to the difference in coefficients of thermal expansion between the coating and the quartz as the tube cooled. This white coating is
probably either Al2O3 or AlPO4. I measured the thickness of a sliver of the coating and found it to be .05 mm; the tube wall is 1.5 mm. The last photo
is of the temp probe beside the same model of a new probe. Things got pretty toasty in there. I will probably repeat the experiment in the near future
using CO2 as the sweep gas.




|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ Strepta:
The coating must be alumina, possibly with some unreacted phosphate. I've been fooling around with trying to coat things with alumina as a by-product
of reactions, you've inadvertently succeeded!
CO2 is likely to be an oxidiser for Al in these 'toasty' conditions but whether the Al will favour being oxidised by the CO2 or by the metaphosphate
depends on the chemical equilibria reigning.
Cheap argon welding gas is still your best bet as an inert atmosphere but have you considered not using an inert atmosphere at all? The amount of
oxygen contained in your assembly is really quite small: at STD conditions 22.4 L of O2 is still only about 32 g (1 mole). Without inert gas, some P
would be lost to oxidation by O2 but once the small amount of O2 has been scavenged, your P is good to go!
Which type of thermocouple did you use and where did you get them?
|
|
|
Strepta
Harmless

Posts: 44
Registered: 6-5-2004
Member Is Offline
Mood: No Mood
|
|
@blogfast:
I've used the CO2 before even with elemental Al in the tube. While some may have been consumed, the amount did not appear to be significant.
The gas is used solely for sweeping the product away from the reactants and making recovery/harvesting of any P4 easier.
I favor CO2 because of ease of fixturing, availability and cost. A reducing gas such as H2 could also be used, and I did use it (produced from an
electrolysis cell)in a reaction at 700 C described somewhat upthread. Some are concerned with using flammable gas at elevated temps but the volume in
these experiments is so small that even a tube failure should not produce more than a loud pop.
The probe is an Omega TJ36-CAXL-18G-12.
|
|
|
| Pages:
1
..
16
17
18
19
20
..
60 |