| Pages:
1
..
16
17
18
19
20
..
25 |
OneShot
Harmless

Posts: 21
Registered: 19-3-2011
Member Is Offline
Mood: No Mood
|
|
nurdrage you have msn? I gotta talk to you thanks
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Warnings about anhydrous hydrazine
The method of initially refluxing hydrazine hydrate with NaOH in as was given in Brauer led to a severe explosion with larger batches (~1kg). Later
editions of Georg Brauer's book use less dangerous ammonolysis of hydrazine salts but hazard of this procedure is not mentioned in the later editions.
Hydrazine that's near anhydrous is extremely dangerous to distill (explosion has occurred even in inert atmosphere! But in air it's a
deathwish in the making, also results of detonating hydrazine with blasting caps in the ballistic mortar test showed the explosion in an air
atmosphere is 11 to 13 times stronger), one would really have to prepare and brace for detonation occuring anytime. Plants which make hydrazine were
designed to handle explosion. I've read about several detonations that occurred from distilling anhydrous hydrazine for no apparent reason! Vacuum and
inert gas, clean glass equipment, very controlled heating, and small amounts below 100g are a must for starters. Saftey shield and similar protection
can not be ignored. Entrainers are used to make detonating hydrazine vapor diluted but this can still get dangerous (entrainers are used in
dehydration distillation), blanketing fluids used in distillation have been used for the same purpose.
I've thought about distilling anhydrous hydrazine, because it looked simple but after some research I've just scrapped the idea. More info in:
1) Hydrazine and Its Deriatives by E. Schmidt. 2nd ed. pg.853; pgs. 95-96; pg.99. The whole book basically.
2) Gmelin's Handbuch. 8 Auf. N 314 and Berichte 37, Stollé and Hofmann, [1904] 4524.
3) Explosive Properties of Hydrazine by U.S. Bureau of Mines.
4) Bretherick's Handbook, 6th ed., 4520.
Also hydrazine (and its hydrate) strongest consistent adverse toxic effects are eye (cornea) damage and fatty liver degeneration (E. Schmidt book, pg.
900). Hydrazine is a blood poison since it hemolyzes red blood cells. Carcinogenicity is inconclusive to weak.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Single inhalation exposure to hydrazine hydrate caused liver effects in a Chinese worker, only a few hours after exposure: http://occmed.oxfordjournals.org/content/57/7/535.full Read it and weep.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Formatik  | | Single inhalation exposure to hydrazine hydrate caused liver effects in a Chinese worker, only a few hours after exposure: |
Thank you for this information. Although I don't intend to make anhydrous hydrazine, I probably have been too causal even when making hydrazine
sulfate (chlorourea and Hofmann degradation methods). I did attempt to make 25% hydrazine but because of equipment limitations could only make 3%,
which worked just as well.
I tend to rely on my nose to warn me when to turn on the hood fan. It seems that in the case of hydrazine, and likely many other compounds, that
could lead to serious illness.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Magpie  | | Thank you for this information. Although I don't intend to make anhydrous hydrazine, I probably have been too causal even when making hydrazine
sulfate (chlorourea and Hofmann degradation methods). I did attempt to make 25% hydrazine but because of equipment limitations could only make 3%,
which worked just as well. |
Well, hydrazine forms an azeotrope with water having 71.5% N2H4 at 120.6 C and 771.8mmHg, so in theory all one would have to do to concentrate it is
distill off water. Hydrazine hydrate is much less dangerous to distill. I did this with 35% aq. N2H4 in a copper apparatus cleaned with aqueous
thiourea then water, and distilled on a hotplate over 3 hours to get a fuming hydrazine. I'm not sure how strong it is, but it is significantly
stronger, because it fumes in outside air (35% N2H4 doesn't fume at all) and when it is dripped onto a small pile of KMnO4, it sizzles and spatters
vigorously creating a beige mass and a fireball, and potentially even igniting tissue (35% N2H4 does the same but gives no fireball, or ignition),
even though the liquid doesn't ignite by flame (hydrazine and esp. hydrate have high flash points).
| Quote: | | I tend to rely on my nose to warn me when to turn on the hood fan. It seems that in the case of hydrazine, and likely many other compounds, that
could lead to serious illness. |
I do take precautions, and eat healthier than most normal people and take supplements to make up for my exposure. But I still tend to get too
comfortable and have to remind myself constantly how toxic the material is.
[Edited on 13-4-2011 by Formatik]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I just did a rough titration of the fuming stuff with phenol red and it is around 57.4% N2H4 (around 90% N2H5OH).
|
|
|
bdbstone
Harmless

Posts: 18
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
I try to make some Hedrazine sulfate using NurdRage method and it seems I have the same problem as user 'Zinc' before.
Since this is the first time, I was doing this, I divided all the amount by 5times, use 50ml 25% ammonia, 20ml MEK and for bleach there was a label
that it contains 43grams in a 1L bottle, I calculated that to be around 4.77% so a 78ml was used. There is also another label on the other side of the
bottle: "Ingridients: <5% of bleach substance based on chlor, perfumes". All the chemicals were technical grade, including ammonia so there was no
purity written on them.
First I mix the ammonia and MEK, for about 1 minute, then let it stand for a while. The two chemicals separated, so I mix with glass rod some more and
let it stand. The two chemicals were still separating, no matter how much I mix them, so I continue with another step. I transfered them to another
bottle and added slowly bleach to the mix. There was definitelly some bubbles and foam, every time I add bleach. The whole process of adding have go
for around 10-20 minutes. I transfered it all to the measuring cylinder and let it stand, however in more than ten hour period there still isn't any
sign of separation going on.
I would love to try that with toulouene, but the thing is I don't have any. I am still wondering what was the problem here.
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
in your case and in zinc's case less than 5% hypochlorite concentration was used.
the problem just seems to be one of concentration and dilution.
do you have any other non-polar liquid for which to perform an extraction?
i haven't tried this, so i don't know if it works, but maybe adding more MEK (after adding the hypochlorite) might saturate it enough that a mixture
of MEK and Azine separate out.
Exactly how much? i have no idea.
|
|
|
bdbstone
Harmless

Posts: 18
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
I will try that out.
After watching your video again, I have also find out, that you use 30% solution. Since my ammonia solution is 25% percent solution, I should use 60ml
instead of 50ml. Maybe that was also the problem..
I will try it again today, with another batch, but I will measure the liquids more carefully.
Thank you.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Try using urea instead of ammonia. That could make it less diluted.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Anyone tried satured sol. of urea in water + calcium hypochloride in small batch to make hydrazine hydrate?
2CO(NH2)2 + Ca(ClO)2 => 2CaCl2 + 2CO2 + 2N2H4
Edit i have thinked of something that i will test , witch use a catalyst:
First stage:
carbamine peroxide + trimethylamine(catalyst) → urea + water + trimethylamine n-oxide
(NH2)2CO.H2O2 + (CH3)3N →(NH2)2CO + H2O + (CH3)3NO
second stage:
trimethylamine n-oxide + urea → carbon dioxide + hydrazine + trimethylamine
(CH3)3NO + (NH2)2CO → CO2 + N2H4 + (CH3)3N
overal process:
carbamine peroxide + trimethylamine(catalyst) → carbon dioxide + water + hydrazine + trimethylamine
(NH2)2CO.H2O2 + (CH3)3N → CO2 + H2O + N2H4 + (CH3)3N
[Edited on 27-4-2011 by plante1999]
I never asked for this.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
gentlemen i'm trying to make hydrazine sulfate via nurd rage method. i got all the liquids in place but never noticed any strong bubbling when i would
add bleach to MEK /ammonia solution.i let the whole thing stand over night as it never seperated into two phases and this morning i got about 50ml of
the now orange solution and added bleach and i did see the fizzing. i added more bleach to the rest of the solution and it fizzed and turned white
again as i stirred so i think my bleach was weak. my question is if sodium hypochlorite density is 1.11 g/cm3 would this mean that 1ml=1.11 grams? at
this point i'm just adding bleach as i see fizzing while i stir but if this does not work then i will get better bleach and proper proportions.i will
be using 6% bleach. thank you.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I found that as my 12% bleach deteriorated to 6% the sp gr stayed the same. I assume that as the NaOCl converts to NaCl there is no effect on
density. To get the true strength I titrated with standardized HCl.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
magpie i dont understand, in nurd rage's method it calls for 300 grams of 6% sodium hypochlorite and i was thinking if 300ml of 6% bleach was the same
as 300 grams in this case. wikipedia states NaCLO density is 1.11g/cm3, is that for 100% solution? but then a 300 gram solution of 6% NaCLO would be
like a 55 gal. drum worth. i thought density would tell me how much bleach to use. the mixture smells like a 200 proof bacardi that burns your
nose.adding more MEK did cause a two layer separation but i could'nt leave well enough alone and stirred it again.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's a document that will tell you all about aqueous NaOCl, compliments of Rosco Bodine. For questions about NurdRage's procedure it would be best
to address them to him as I haven't tried it.
http://www.sciencemadness.org/talk/files.php?pid=195705&...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cyanureeves  | | magpie i dont understand, in nurd rage's method it calls for 300 grams of 6% sodium hypochlorite and i was thinking if 300ml of 6% bleach was the same
as 300 grams in this case. wikipedia states NaCLO density is 1.11g/cm3, is that for 100% solution? but then a 300 gram solution of 6% NaCLO would be
like a 55 gal. drum worth. i thought density would tell me how much bleach to use. the mixture smells like a 200 proof bacardi that burns your
nose.adding more MEK did cause a two layer separation but i could'nt leave well enough alone and stirred it again. |
300g of bleach should be at most 300mL, less if the density is higher.
You might want re-check your calculations or your spreadsheet for an error somewhere. 55 gallons volume for 300g of a substance would seem to indicate
the density of a gas rather than a liquid.
i picked 300g of 6% bleach because that gives 18g of "pure" NaClO. i designed the rest of the experiment around that molar amount.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
thank you nurd rage. i know bleach from the store is reliable and the MEK is too ,but not so my ammonia solution. your glass with MEK looked alot
smaller than mine . i'm using a 600ml kimax glass and 100ml looks like swig,maybe you got huge hands. magpie thank you for the everything you need
to know about NaCLO but was afraid to ask document.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Concerning the set-up I mentioned above: I used all-copper apparatus made from pipes and connections. It was just a simple L-shaped apparatus. The
distillation tube was soldered (tin solder, not lead!) to the connecting joint (piece that connects to the vessel). But the joint piece was not
soldered to the vessel since I was thinking of doing other things with this. So during the distillation I wrapped Teflon tightly around this area then
taped it with a strong tape that can withhold the heat. I filled the vessel with less than half in volume of its capacity with 35% aq. hydrazine
solution (about 50mL). Heating this at an angle (with a clamp and stand) over the hotplate was hot enough to distill off water. But it took a long
time to begin distilling. It would have been better to have started the hotplate heat on high setting to begin with. Wrapping the vessel in an
aluminium foil blanket later helped with the heating process.
But before distillation, it was taking way too long and I got impatient and heated the copper tube with the Bunsen burner (for a few moments), this
caused a portion of the whole solution to come over (this wasn't needed at all, I just got impatient). Then after it started distilling, I distilled
for around 3 hours just using the hotplate. The outside temperature of the lower portion of the copper vessel was around 154 C. But this temperature
was necessary for reasonable distillation. Some hydrazine did come over. The water was collected in a graduated cylinder covered with aluminium foil.
After distilling, and when cold the hydrazine left in the copper vessel was then fuming. There is a rough diagram below, except the tube was much
longer than what is shown and it was more L-shaped. Copper can likely not be substituted by other metals, maybe except silver, because of ignition
point changes. I also washed the entire apparatus with thiourea and then water (prior to distillation). This can most likely be used to make hydrazine
hydrate from hydrazine salts and bases, plus enough water. And then a sand bath ought to be better than a hotplate. Hydrazine hydrate
can be prepared like this, but near anhydrous hydrazine is too dangerous.
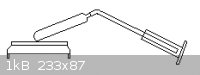
[Edited on 4-5-2011 by Formatik]
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
o.k. now. i googled hydrazine sulfate and read that it can make one mellow and eat like a pig if eaten and hydrazine can make jet fuel. are these
things true? when or how is hydrazine sulfate made flammable? is this set -up the very thing that ties both of my queries?
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
This set-up above doesn't address your post above it. Someone asked about how I made concentrated hydrazine hydrate, and this explains it. Yes, strong
hydrazine hydrate is used as jet fuel. I wouldn't advise eating hydrazine sulfate.
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Is it safe to perform Rosco hydrazine sulfate synthesis under kitchen aspirator? Is there any risk of poisoning?
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Update / correction!
Back in 2004 (wow, 6 years ago) I attempted to synthesize hydrazine by heating urea with nickel to produce nickel carbonyl / hydrazine. My first
experiment used nickel shavings / drillings / pieces all of which were somewhat large and it was done on the small scale in a test tube. My second
experiment was done in a RB with nickel powder and urea....
At least that was the intention. Some time ago I was talking to a member of this forum via messenger and they pointed out that heating nickel oxalate
does NOT necessarily produce nickel metal but nickel oxide.
Here is just one of many papers attesting to this:
http://proj3.sinica.edu.tw/~chem/servxx6/files/paper_13486_1...
There are methods to produce nickel powder from the oxalate, in one instance the oxalate was dehydrated first with heat and vacuum and then decomposed
rapidly at high temp to get nickel metal. But I did not meet this condition and although some nickel may have been formed what I've found is that my
'nickel powder' could have potentially contained little to no elemental nickel. That being the case I would consider the possibility of this reaction
once again open for debate.
I apologize for not being more through in my initial research, but hopefully this will allow someone to try this on the right path.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Bromic acid , you should try to take nickel piece grind it with a lime , blend the shavings with an electric blander and finish with a ball mill , i
only use one time a ball mill for making Fe powder for ferrate and it work after 2-5 week of runing , i made mine from legos....
I never asked for this.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i am dying to see a picture of that!!
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Hydrazine Sulfate Synthesis
I made Hydrazine Sulfate again last week and never got around to posting the few pictures I took. I used Rosco's method in general. I find the process
to be quite robust as I didn't follow all intructions exactly as outlined in the procedure (because of lack of time and laziness), and still got 81.5g
of Hydrazine Sulfate for a half batch. I think my yield has a lot to do with my reagents (probably the hypochlorite), because the first time I made HS
I got a very similar yield and I was extremely sloppy. This time I was much more careful (relatively) to temperatures, reactions time and addition
rates.
Anyway here are a few pictures. The gross brown color is from the black drain cleaner I used to do the final neutralization and precipitate the HS.
The brown color pretty much completely washed away when washing the HS with 70% isopropyl alcohol during filtering/washing.
The first picture was taken sometime during the first 10 minutes, notice with my reagents I have very little foaming. The second picture shows the end
of reaction, very pale yellow color. BTW, that is a 5L erlenmeyer, and a 3" stirbar. The third and fourth show the final neutralization with H2SO4.
The last shows the damp yield. The dry yield taken a couple of days later was measured as 81.5g.
I should have gotten a picture of the primary neutralization with HCl, it was very neat looking, but I forgot to take a picture. A hotplate-stirrer
was used between the first and second pictures, but I forgot to take a picture of that part of the process. The only thing I had on hand to do the
acid additions with was a 25mL burette, so I had to top up the burette quite a few times, not ideal.
    
[Edited on 29-5-2011 by Hennig Brand]
|
|
|
| Pages:
1
..
16
17
18
19
20
..
25 |