| Pages:
1
..
15
16
17
18
19
..
40 |
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
In this part of the reaction this doesn't matter. And on the other reaction vessel they are changed.
Abs. pyridine is in both flask and the reaction goes for 3 days at 90 Celsius where the pyridine is not boiling, so actually it doesn't matter how the
condenser is on the flask.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Rocks & Minerals
It's time for a short geology lesson, boys and girls. Sorry, I don't have a DSLR, just a point and shoot in macro mode. Lighting is mostly halogen.
The pictures may not be the prettiest, but it's overcast this morning and my hands are too shaky to compensate with longer exposures.
Note: Click thumbnails for original full resolution photos.
<table width="804" cellpadding="0" cellspacing="4" align="center"><tr><td><a
href="http://www.medievalsiege.net/bfesser/images/geology/atacamite.jpg" target="_blank"><img
src="http://www.medievalsiege.net/bfesser/images/geology/atacamite_thumb.jpg" /></a></td><td><a
href="http://www.medievalsiege.net/bfesser/images/geology/columbite-tantalite.jpg" target="_blank"><img
src="http://www.medievalsiege.net/bfesser/images/geology/columbite-tantalite_thumb.jpg" /></a></td></tr><tr><td
align="center" valign="top"><a href="http://en.wikipedia.org/wiki/Atacamite" target="_blank">Atacamite</a> <img
src="../scipics/_wiki.png" />, Cu<sub>2</sub>Cl(OH)<sub>3</sub>, from the <a
href="http://en.wikipedia.org/wiki/Type_locality_(geology)" target="_blank">type locality</a> <img src="../scipics/_wiki.png" /> in the
<a href="http://en.wikipedia.org/wiki/Atacama_Desert" target="_blank">Atacama Desert</a> <img src="../scipics/_wiki.png" />,
Chile—a region I hope to visit someday.</td><td align="center" valign="top"><a href="http://en.wikipedia.org/wiki/Coltan"
target="_blank">Columbite-Tantalite</a> <img src="../scipics/_wiki.png" /> (Nb/Ta
minerals)</td></tr><tr><td><a href="http://www.medievalsiege.net/bfesser/images/geology/manganite.jpg"
target="_blank"><img src="http://www.medievalsiege.net/bfesser/images/geology/manganite_thumb.jpg" /></a></td><td><a
href="http://www.medievalsiege.net/bfesser/images/geology/vanadinite.jpg" target="_blank"><img
src="http://www.medievalsiege.net/bfesser/images/geology/vanadinite_thumb.jpg" /></a></td></tr><tr><td align="center"
valign="top"><a href="" target="_blank">Manganite</a> <img src="../scipics/_wiki.png" />, MnO(OH), high purity
crystals</td><td align="center" valign="top"><a href="http://en.wikipedia.org/wiki/Vanadinite" target="_blank">Vanadinite</a>
<img src="../scipics/_wiki.png" /> (Pb<sub>5</sub>(VO<sub>4</sub> <sub>3</sub>Cl) on <a href="http://en.wikipedia.org/wiki/Baryte" target="_blank">Barite</a> <img
src="../scipics/_wiki.png" /> (BaSO<sub>4</sub> <sub>3</sub>Cl) on <a href="http://en.wikipedia.org/wiki/Baryte" target="_blank">Barite</a> <img
src="../scipics/_wiki.png" /> (BaSO<sub>4</sub> </td></tr></table> </td></tr></table>
I hope to post a picture in the future of some Au specks I found on a rock in the Black Hills, SD. I'll need to set up a microscope or powerful
loupe, though. It was a real treat to find a native element!
<!-- top secret project <a href="http://en.wikipedia.org/wiki/Manganese_nodule" target="_blank">Manganese (polymetallic) nodule</a>
<img src="../scipics/_wiki.png" />
<a href="http://www.medievalsiege.net/bfesser/images/geology/manganese_nodule.jpg" target="_blank"><img
src="http://www.medievalsiege.net/bfesser/images/geology/manganese_nodule_thumb.jpg" /></a>
The manganese nodule may not be impressive to any of you, but it's beatiful in my eyes. For me, it holds special significance, mainly because I
collected it myself by trespassing on privately owned land (destroyed a shirt while prone-crawling under the barbed-wire perimeter fence) <a
href="http://wikimapia.org/#lat=43.8059734&lon=-99.4048656&z=18&l=0&m=b" target="_blank">near Oacoma, SD</a> <img
src="../scipics/_ext.png" />. I think it's cool to hold something that once formed at a fumeral near a mid-oceanic ridge. I plan to return to the
locale to collect more specimens, this time with landowner permission. -->
[Edited on 7/23/13 by bfesser]
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Hey guys, I'm plugging for this thread as a shout out for some awesome pictures! I am re-tackling the book project, made by home chemists, for home
chemists.
https://www.sciencemadness.org/whisper/viewthread.php?tid=25...
Give me a hand guys and check out the web page of the book as well!
http://www.bromicacid.com/bookprogress.htm
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
I think you mean mineralogy.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
turd; seriously? Who are you trying to impress with your attempted correction?
But if you want to go there . . .
I <em>meant</em> <a href="http://en.wikipedia.org/wiki/Geology" target="_blank">geology</a> <img src="../scipics/_wiki.png"
/>; as I wrote it. <a href="http://en.wikipedia.org/wiki/Mineralogy" target="_blank">Mineralogy</a> <img src="../scipics/_wiki.png"
/> is often considered a <a href="http://en.wikipedia.org/wiki/Category:Subfields_of_geology" target="_blank">subfield of geology</a>
<img src="../scipics/_wiki.png" />. <!-- top secret project Mn <a href="http://en.wikipedia.org/wiki/Nodule_(geology)"
target="_blank">nodules</a> <img src="../scipics/_wiki.png" /> are also <a href="http://en.wikipedia.org/wiki/Rock_(geology)"
target="_blank">rocks</a> <img src="../scipics/_wiki.png" /> and would fall outside the domain of mineralogy (in this context). -->
In the future, please keep such pedantic and asinine comments to yourself.
[Edited on 7/23/13 by bfesser]
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Keep it clean gentlemen(women)!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Yes, why not? And I'm so above having to impress or offend anybody.
| Quote: |
But if you want to go there . . .
I <em>meant</em> <a href="http://en.wikipedia.org/wiki/Geology" target="_blank">geology</a> <img src="../scipics/_wiki.png"
/>; as I wrote it. <a href="http://en.wikipedia.org/wiki/Mineralogy" target="_blank">Mineralogy</a> <img src="../scipics/_wiki.png"
/> is often considered a <a href="http://en.wikipedia.org/wiki/Category:Subfields_of_geology" target="_blank">subfield of geology</a>
<img src="../scipics/_wiki.png" />. Mn <a href="http://en.wikipedia.org/wiki/Nodule_(geology)" target="_blank">nodules</a> <img
src="../scipics/_wiki.png" /> are also <a href="http://en.wikipedia.org/wiki/Rock_(geology)" target="_blank">rocks</a> <img
src="../scipics/_wiki.png" /> and would fall outside the domain of mineralogy (in this context). |
Well, English speaking countries tend to have some peculiar customs[1]. So maybe they classify mineralogy and petrology as a subfield of geology - but
in my neck of the wood this is certainly not the case, just like biology is not considered part of chemistry and chemistry is not considered a part of
physics. I converse with both - geologists and mineralogist - and apart from belonging to the same faculty (earth sciences or geosciences) they have
little in common. Different analytic methods, different field trips, different conferences, etc. And certainly they would not consider one a subfield
of the other.
Edit: When I get a lesson in geology I expect pictures of outcrops (c.f. https://www.google.com/search?q=outcrop&tbm=isch). Of folds, cinder cones, etc. The pictures you posted are clearly for a mineralogy
lesson (and yes, even the Mn thing will be typically found in a mineralogical collection). That's all I said.
[1] Like publishing full name and photo of suspects and convicts from people who pissed against a tree to child molesters. Or believing that 16 year
olds are responsible enough to drive a car, but they may only decide to relax with a beer with 21. No wonder they're all a little bit tense. 
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: fixed
broken image(s)]
[Edited on 7/9/13 by bfesser]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Fair enough, turd. I concede your point.
An <a href="http://en.wikipedia.org/wiki/Outcrop" target="_blank">outcrop</a> <img src="../scipics/_wiki.png" /> exhibiting <a
href="http://en.wikipedia.org/wiki/Fold_(geology)" target="_blank">folding</a> <img src="../scipics/_wiki.png" /> (<a
href="http://en.wikipedia.org/wiki/Anticline" target="_blank">anticline</a> <img src="../scipics/_wiki.png" /> most clearly visible) in
the <a href="http://en.wikipedia.org/wiki/Black_Hills" target="_blank">Black Hills, SD</a> <img src="../scipics/_wiki.png" />.
Exact location forgotten (I didn't take the photo–I was unwittingly in it).

Sorry, it's definitely not a "pretty picture".
[Edited on 7/9/13 by bfesser]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
The photography snobs will definitely complain (the lighting! the detail! the composition!), but I think it's a nice picture. It conveys an
interesting situation.
[Edited on 6-5-2012 by turd]
|
|
|
Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
here are some photos of the synthesis of nitrotetrazole
http://www.explosci.com/nitrotetrazole-synthesis/
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by turd  |
The photography snobs will definitely complain (the lighting! the detail! the composition!), but I think it's a nice picture. It conveys an
interesting situation.
[Edited on 6-5-2012 by turd] |
If you were referring to me (as the one who started with the complaints), then no, I don't think this is a bad photograph.
It's not a great photograph, but it's not a piece of trash. It's interesting and the colors are nice, too.
I hate photography snobs. They often fixate on written rules and don't appreciate the creativity. They'll often annoy people with "the lines aren't
paralell", "the horizon isn't horizontal" etc. even when it's unneccessary.
Complaining about obviously bad photographs on a thread meant for depositing "pretty pictures" is not snobbism. It's simply a kick in the butt to turn
on the creativity mode.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Demanding artistic creativity from a bunch of autistic nerds may not be snobbish, but probably is futile. 
After the short excursion into geology/pretrology/mineralogy, back to some chemistry: 2,5-dimethoxy-benzaldehyde and 2,5-dimethoxy-beta-nitrostyrene.
Which is which is obvious. The last picture is taken under a polarizing filter - for some reason it's impossible to catch the amazing views with a
CCD/CMOS camera.  At least one sees a little bit of dichroism. At least one sees a little bit of dichroism.
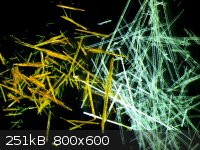 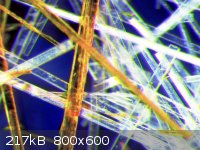  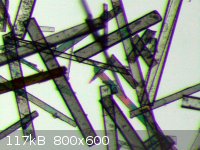
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Copper Acetate
As promised, here are the crystals are 'cleaning them' with glacial acetic acid. I am planning on using one of these as a seed crystal and going after
much larger ones. More pics to come!

Also, here is the BaCl2 after filtering and rinsing. They are so clear, they may be tough to see.

|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Those are nice pictures, but I think that with all pictures, the viewer should have a sense of scale. I understand that with microscope shots,
including scale is difficult if not impossible, but you could at least include the magnification
I have some copper acetate I prepared two years ago. I never bothered to grow crystals because the powder dissolves faster, and I'm an impatient
person Since I haven't used it for anything for a very long time, I'll grow
crystals and post pictures. Since I haven't used it for anything for a very long time, I'll grow
crystals and post pictures.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Quote: Originally posted by White Yeti  | Those are nice pictures, but I think that with all pictures, the viewer should have a sense of scale. I understand that with microscope shots,
including scale is difficult if not impossible, but you could at least include the magnification
I have some copper acetate I prepared two years ago. I never bothered to grow crystals because the powder dissolves faster, and I'm an impatient
person Since I haven't used it for anything for a very long time, I'll grow
crystals and post pictures. Since I haven't used it for anything for a very long time, I'll grow
crystals and post pictures. |
True, eh maybe next time with the larger crystals. I just like the geometry of these! 
Be sure to post yours when you're done!
[Edited on 9-5-2012 by sargent1015]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | Those are nice pictures, but I think that with all pictures, the viewer should have a sense of scale. I understand that with microscope shots,
including scale is difficult if not impossible, but you could at least include the magnification |
Actually, any modern microscope software will do it for you. On older microscopes you used transparent foil with a well defined grid on it, and there
also were some cute micro-rulers. Some people use a ruler printed on the ocular and a magnification to size per unit table.
Should someone care: the largest 2,5-diMeO-PhCHO needles were around ~2 cm, in the picture above ~1.5 cm.
@sargent1015: the BaCl2 picture really is terrible. White on white - is this some joke? It reminds me of those "Barcelona by night" postcards.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I didn't know software existed to make measuring easier.
The only device I've ever used to measure things under a microscope was a tiny ruler with microscopic demarcations.
Since it was such a pain to position and read, standard operating procedure was to cite the magnification.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Quote: Originally posted by turd  | Demanding artistic creativity from a bunch of autistic nerds may not be snobbish, but probably is futile. 
After the short excursion into geology/pretrology/mineralogy, back to some chemistry: 2,5-dimethoxy-benzaldehyde and 2,5-dimethoxy-beta-nitrostyrene.
Which is which is obvious. The last picture is taken under a polarizing filter - for some reason it's impossible to catch the amazing views with a
CCD/CMOS camera.  At least one sees a little bit of dichroism. At least one sees a little bit of dichroism.
|
Wow nice crystals!!! They look almost psychedelic 
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|

A little gif from trimethylborate flames a couple of friends and I made a year ago..
Edit: Edited the gif a with the proper delay between each frame (150ms).
Edit: Another one, though not animated..

[Edited on 11-5-2012 by bahamuth]
[Edited on 11-5-2012 by bahamuth]
[Edited on 11-5-2012 by bahamuth]
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Magnesium Chloride needle-like crystals. Awful yield, but fantastic purity. Anyone have a better method for crashing these out of solution and washing
them?

|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
That picture is fuzzeh. Try using macro mode. Also, try not to get Endimion17 too upset about form, shape, perspective, colour, balance and texture or
lack thereof 
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Aw man it is fuzzy... Crap... 
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
turd, bahamuth, AWESOME 
[Edited on 18-5-2012 by Endimion17]
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
Here's a bit of copper acetate I made recently.

[Edited on 21-5-2012 by liquidlightning]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Beautiful crystals. I am trying to make some home-made sodium nitrite...
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
| Pages:
1
..
15
16
17
18
19
..
40 |