| Pages:
1
..
14
15
16
17
18
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  |
Indeed, but I was talking about the first two in that thread. They're much more robust IMO, especially if aga is having issues with the ethanol
chlorination. |
My impression is that his problem is isolation, not the actual formation of the chloral hydrate. Distilling off some unreacted EtOH
as water azeotrope could do wonders for the chloral hydrate crystallising out, after some of its solvent has been removed and its concentration
increased.
After chlorination, distil at AP to about 90 C overhead. Cool and chill. Collect crystals.  (hope springs eternal) (hope springs eternal)
What do you think?
[Edited on 25-1-2016 by blogfast25]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Will chloral hydrate oxidize to trichloroacetic acid? My only concern is that the acetal might inhibit the oxidation. Distilling the hydrate with
sulfuric acid might be necessary prior to the oxidation. Other than that, the workup looks fine, just be aware that chloral hydrate has a significant
vapor pressure at the BP of erhanol, and some will be carried over.
[Edited on 1-25-2016 by gdflp]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | Will chloral hydrate oxidize to trichloroacetic acid? My only concern is that the acetal might inhibit the oxidation. Distilling the hydrate with
sulfuric acid might be necessary prior to the oxidation. Other than that, the workup looks fine, just be aware that chloral hydrate has a significant
vapor pressure at the BP of erhanol, and some will be carried over.
[Edited on 1-25-2016 by gdflp] |
No, I meant the oxidation of chloral (trichloroacetaldehyde) with HNO3 to trichloroacetic acid. So the conversion of
chloral hydrate to chloral (real confusing names!) would definitely still be necessary, with conc. H2SO4.
[Edited on 25-1-2016 by blogfast25]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by blogfast25  | [rquote=436660&tid=15]
No, I mean oxidation of chloral (trichloroacetaldehyde) with HNO3 to trichloroacetic acid. So the conversion of chloral
hydrate to chloral (real confusing names!) would definitely be necessary, with conc. H2SO4.
[Edited on 25-1-2016 by blogfast25] |
They certainly are. Sounds like you already thought of that then, so looks good.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
There's also this patent on the chlorination of anh. EtOH with Cl2, in the presence of an immersed 250 W light bulb:
http://www.freepatentsonline.com/2478152.pdf
Chlorination times of about 7 h at 30 C, with HCl evolution starting at 4 h.
I wonder what reaction times were used by aga...
And then there's this (from that patent):

So the author claims that in anh. EtOH the reaction is to chloral alcoholate:
2 C2H5OH + Cl2 ===> CCl3CH(OH)OC2H5 + HCl
<hr>
Pfffft... finally got to the bottom of it, I think.
In anh. EtOH the principal reaction appears to be:
2 C2H5OH + 2 Cl2 ===> CCl3CH(OH)OC2H5 + HCl
CCl3CH(OH)OC2H5 = chloral ethanolate (aka chloral alcoholate), MP about 23 C.
On reaction with an excess conc. H2SO4, this gives the desired chloral (trichloro acetaldehyde):
CCl3CH(OH)OC2H5 + H2SO4 ===> CCl3CHO + C2H5HSO4
+ H2O
C2H5HSO4 = ethyl hydrogensulphate
Low boiling ethyl chloride, ethanol water azeotrope (and others) can then be distilled off, before the chloral (trichloro acetaldehyde) comes over at
98 C.
See also:
https://docs.google.com/viewer?url=patentimages.storage.goog...
[Edited on 25-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice work bloggers.
The conclusion i came to after reading up on the Haloform Reaction mechanism is that in the the presence of a Base, then Haloform is what occurs:
OH<sup>-</sup> causes haloform results.
Water is probably acting as a Base in wet ethanol, which is why no solids formed when chlorinating - the wrong products happen, and are (thankfully!)
not white solids.
The Time between starting chlorination and the appearance of cloudiness, then obvious solids is not long, just a few tens of minutes, as was seen in
the first inverted funnel experiment.
Happily there is around 400ml of 96% ethanol today, after a lot of careful distilliation.
The calculations for the entire series from ethanol to TCCA were awesome.
We need very little dry ethanol to arrive at a useful amount of the TCA catalyst required.
To chlorinate 100g of ethanol, the Cl2 needed has to come from a quarter of a Kilo of TCCA !
50g dry ethanol will eventually give (if all goes well) more than sufficient TCA for the purpose of making turpineol.
Remember that stuff ?
Vaguely recall it being the original target compound 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
The Time between starting chlorination and the appearance of cloudiness, then obvious solids is not long, just a few tens of minutes, as was seen in
the first inverted funnel experiment.
Vaguely recall it being the original target compound  |
Well, considering what I've been reading, that sounds like a really short time!
Target compound clearly in sight, no worries.
As regards a reaction mechanism, here's a tentative post hoc candidate.
The chlorination itself is likely to be via free radical mechanism. You can read up on these here:
http://www.chemguide.co.uk/mechanisms/freerad/ch4andcl2.html...
I'm assuming the chlorination proceeds to 1,2,2,2-tetrachloroetan-1-ol, then the remaining alcohol acts as a Lewis base, as shown below:
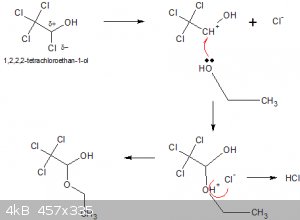
With water instead of ethanol the adduct would be chloral hydrate, with ethanol chloral alcoholate, as suggested in one of the patents linked to.
The overall stoichiometry is:
2 C2H5OH + 4 Cl2 === > CCl3CH(OH)OC2H5 + 5 HCl
Dehydration with conc. H2SO4 yields chloral (trichloroacetaldehyde) and ethyl hydrogensulphate.
[Edited on 25-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
That is what i observed, doing it.
Short, Long, i'm the man with the Chlorine (cheers Bert !).
The proposed reaction mechanism looks Odd.
Firstly, why is the Cl2 disassociating at all ?
It is Really happy as Cl-Cl.
Is anhydrous ethanol capable of solvating Chlorine ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
That is what i observed, doing it.
Short, Long, i'm the man with the Chlorine (cheers Bert !).
The proposed reaction mechanism looks Odd.
Firstly, why is the Cl2 disassociating at all ?
It is Really happy as Cl-Cl.
|
Free Cl* radicals can be formed by UV photons cleaving the molecules:
$$Cl_2(g) + h\nu \to Cl*(g)$$
The radicals then 'trickle down' the mechanism chain, so you don't really needed masses of them.
So keep the lights on, especially black lights of TL tubes or saver bulbs!
'Happiness' is relative: systems strive to lose Gibbs Free Energy, remember?
<hr>
Oh and properties of chloral alcoholate:
http://www.drugfuture.com/chemdata/chloral-alcoholate.html
MP apparently 47.5 C.
[Edited on 26-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | The overall stoichiometry is:
2 C2H5OH + 4 Cl2 === > CCl3CH(OH)OC2H5 + 5 HCl
Dehydration with conc. H2SO4 yields chloral (trichloroacetaldehyde) and ethyl hydrogensulphate. |
Good stuff ! Working out what actually happens has been rather difficult.
For that second reaction i could not get the masses to balance unless 1 molecule of water was also produced.
Here is a spreadsheet with the five reactions calculated out and chained, starting with the required amount of TCA and working backwards.
Attachment: TCA Route Calcs.xlsx (13kB)
This file has been downloaded 577 times
The TCA required is changed in the yellow box at top-right, then the quantities of chemicals used/made is calculated.
An ethanol mass of 200g (= last attempt) would theoretically produce 54g of TCA, requiring a whopping 209 litres of chlorine gas !
That's a lot of bubbles and bubbling time.
This is where the cock-up happened : starting to react stuff before doing the calculations.
15g of TCA might be a safer quantitiy to aim for, although i'm not sure that it would be enough.
Based on the TCA process ratios, 15g TCA would convert 28g of alpha-pinene.
[Edited on 26-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nice work on the spread sheet! Failing to plan is planning to fail.
This reference confirms the product of chlorination is chloral alcoholate but suggests a different route to the one above.
1. Oxidation of ethanol to ethanal by chorine:
CH3CH2OH === > CH3CHO + 2 H<sup>+</sup> + 2 e
Cl2 + 2 e === > 2 Cl<sup>-</sup>
2. Formation of hemiacetal:
Reaction of ethanal with ethanol (nucleophilic attack by EtOH on carbonyl carbon):
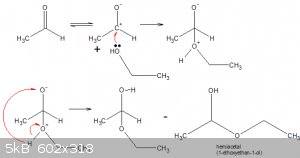
3. Chlorination of hemiacetal:
Presumably by radical chlorination, the three H on the left C are substituted, because they are the most polarised due to the induction by the
O-atoms.
The stoichiometry remains 2 ethanol + 4 chlorine = chloral alcoholate + 5 HCl
Alternatively one could imagine chlorination of the ethanal to chloral (trichloroethanal), with subsequent reaction of the chloral
with ethanol to chloral alcoholate.
[Edited on 26-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Stoichimetry is all wrong for the Cl2 generator calculations - my pool shock stuff is Na-DCCA.2H2O, not TCCA ! 
Those pesky small-print labels ...
Local version of the spreadsheet altered.
How much Terpineol are we aiming for, so i can work out how much TCA is needed ?
22g TCA requires almost an entire half-kilo jar of Na-DCCA, 1 kilo of 20% HCl (both really cheap here) generating 85.4 litres of Cl2
(scary) to gas 82g of ethanol.
As for Time, 85.4 litres = 85,400,000 mm<sup>3</sup>.
If a bubble is about 4mm across, that's approximately a volume of 33.5 mm<sup>3</sup>/bubble.
At 5 bubbles a second, the total gassing time would be 5.9 days, assuming each bubble is 100% absorbed !
No wonder TCA is expensive.
200ml of azeo ethanol is being dried over some K2CO3 that was bought and forgotten about.
The K3PO4 produced for the purpose did not work. Not all of it dissolves rapidly in water, so it is Highly contaminated.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Considering we still need to hydrogenate it, I'd say about 10 g. But I wouldn't worry too much about that: try and get whatever is possible, you can
always scale-up later.
I still think commercial CaO, thoroughly dried before use and stood overnight in azeo EtOH is the cheapest and best route to anh. EtOH.
<hr>
A 'chlorinator' of this type might work better because of the longer bubble path than an RBF:
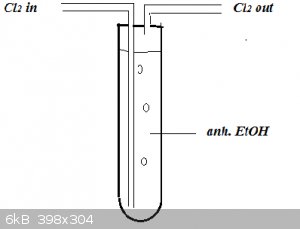
Put a strong spotlight on it!
[Edited on 26-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cement !
All this time searching for a local supplier of CaO and there has been a pile of 25kg bags of Cement in the car park !
Major "Doh !" moment just happened.
Will try it in the morning: dry azeo EtOH with Cement.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Cement !
All this time searching for a local supplier of CaO and there has been a pile of 25kg bags of Cement in the car park !
Major "Doh !" moment just happened.
Will try it in the morning: dry azeo EtOH with Cement. |
Ooopsie. More a "Dang !" moment, IMHO.
Something got lost in translation?
It does contain CaO: I once made KOH with it.
Probably best trying it in a plastic container? 
Expect the cement to contain about 40 w% of active CaO, the rest being tied up as silicates and aluminates.
[Edited on 31-1-2016 by blogfast25]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Everyone,
Today I successfully ran a Grignard to make 3-methyl-heptan-3-ol. Please see it in prepublications and let me know what you think and if there are any
ways I could revise it!
-SH
http://www.sciencemadness.org/talk/viewthread.php?tid=65439#...
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Quote: Originally posted by aga  | Cement !
All this time searching for a local supplier of CaO and there has been a pile of 25kg bags of Cement in the car park !
Major "Doh !" moment just happened.
Will try it in the morning: dry azeo EtOH with Cement. |
Any update?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
With aga now in self-imposed exile there won't be any, anytime soon I think.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
"With aga now in self-imposed exile there won't be any, anytime soon I think."
This reminds me of the "camphor attempt". ;-)
I found another route to alpha-terpineol:
https://www.researchgate.net/publication/269879454_FeIII-cat...
It utilizes Fe(NO3)3 and hydrogen peroxide in ethanol. This would at least eliminate working with those highly toxic substances.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: |
In this work, an novel and environmentally benign Fe(III)-catalyzed terpinyl derivatives synthesis using hydrogen peroxide in alcohol solutions (i.e
methyl, ethyl, propyl, isopropyl and butyl alcohols) was investigate. β-pinene is the main component of turpentine oil and was the model-substrate
selected. High conversions (ca. 90 %) and combined selectivities for the α-terpineol and terpinyl alkyl ethers (ca. 70-73 %) were obtained, when
Fe(NO)3 was the catalyst. The role of each component catalyst system was studied. The use of biodegradable and renewable origin solvent (ethyl
alcohol), added to an inexpensive and little toxic catalyst and a green oxidant are main positive features of this process. |
Acc. many sources, alpha-pinene is the most important component of commercial (so-called "distilled") turpentine.
Worth bearing in mind though, that work. Thanks for that!
[Edited on 25-8-2016 by blogfast25]
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Of course alpha-pinene is the most abundant, interestingly the error is only found in the abstract on researchgate, while it's correct in the paper.
I wouldn't rule out the reaction works as well for alpha-pinene. The only difference being the energetically less favored position of the double bond
in beta-pinene, which is rearranged anyway, probably by interaction with Fe(NO3)3.
| Quote: | | Worth bearing in mind though, that work. Thanks for that! |
You're welcome!
Edit:
Thymus caespititius essential oil contains about 35 % a-terpineol.
http://www.sciencedirect.com/science/article/pii/S0926669014...
[Edited on 25-8-2016 by Alice]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Alice  |
I wouldn't rule out the reaction works as well for alpha-pinene. The only difference being the energetically less favored position of the double bond
in beta-pinene, which is rearranged anyway, probably by interaction with Fe(NO3)3.
|
As so often these reactions produce a spectrum of reaction products, often hard to separate. That doesn't make things easier at the home lab level.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Possibly, yes. Nevertheless I would be more concerned about the double bond of alpha-pinene to be less susceptible to the lewis acid as it is less
exposed and more stable.
I'll take out one example which seems to have a favorable result:
5 mmol beta-pinene, 5 mmol H2O2, 0.21 mmol Fe(NO3)3 (0.042 eq.) in 15 ml ethanol at 333 K for 4 h yields at 73% conversion:
41% alpha-terpineol
9% alpha-terpineol ethyl ether
20% crap
Meanwhile, I found a reliable source for pure alpha-terpineol:
https://shop.perfumersapprentice.com/p-6228-terpineol-alpha....
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Kremer Pigmente DE also sells it, IIRW, for European buyers.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Unfortunately Kremer sells CAS 8000-41-7 which is an undefined mixture of terpineol isomers. Only alpha and delta-terpineol are suitable as
1,1-methyl alcohols, whereas beta and gamma are not.
http://www.sigmaaldrich.com/catalog/product/aldrich/w304506
|
|
|
| Pages:
1
..
14
15
16
17
18
19 |