| Pages:
1
..
14
15
16
17
18
..
24 |
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
It took you several tries? That's strange, because I was able to seperate terbium from the contaminated mixture in one go. But I had far less iron in
solution than you had.
I have magnet soup of my own that I need to process.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Personally I think it's possible to get the iron level down to almost 0 in one single purification, provided most of the iron is still in the ferrous
state and you maintain high acidity all throughout the process.
But that's not really based on experience. When you precipitate the RE double sulphates from a sea of ferrous sulphate (Nd magnet soup), some
co-precipitation of the FeSO4 may well be unavoidable...
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
blogfast25, the differences in solubility between neodymium and terbium sulfates (terbium sulfate is far less soluble) may account for the observed
differences. However, I need to find out exactly how much iron is needed to quench Tb fluorescence in order to set an upper limit on Fe contamination.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | | blogfast25, the differences in solubility between neodymium and terbium sulfates (terbium sulfate is far less soluble) may account for the observed
differences. |
B&F, we're talking potassium Ln double sulphates here, not simple Ln (III) sulphates. I don't see how the solubility of the latter could influence
the degree of separation from iron.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
No photos of the process, sad to say. If I ever make it all the way to neodymium metal, there's absolutely a video in store when the process is
perfected. It may be worthwhile to post one just getting the Nd separated (my progress up to this point).
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by MrHomeScientist  |
No photos of the process, sad to say. If I ever make it all the way to neodymium metal, there's absolutely a video in store when the process is
perfected. It may be worthwhile to post one just getting the Nd separated (my progress up to this point). |
Definitely would be a worthwhile post!
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I tried adding some potassium sulfate to my magnet nitrate mix, but barely any neodymium sulfate precipitated. After adding in some sulfuric acid to
keep the pH up and halving the volume of the solution, still nothing precipitated. I then added some more potassium sulfate, and something did
precipitate - and it redissolved after a few minutes! This neodymium does NOT want to leave solution, even after being subjected to yet another round
of heating.
The concentration of all the iron and neodymium must be nearing 5 molar now, and that's a very conservative estimate. I don't have much time, nor can
I keep the solution so I really might just have to dispose of the solution and use another magnet.
[edit] wait, there may be something I can do to save the solution...
[Edited on 10.6.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Just an off the top of my head hypothesis, but mixing anoins might be "confusing" the precipitation. You could try dropping out everything as
hydroxides, redissolving in sulfuric acid, and trying again to make the double sulfate. Perhaps in your case there just isn't enough sulfate around?
As for the earlier comment that it took me several tries, I think that was because of faulty method. Remember that I first started with "magnet
sulfate" solution and added a saturated solution of K2SO4 to this. I got a good amount of precipitation, but this was heavily
contaminated with iron due to, as blogfast pointed out, the addition of extra water changing the pH. I then returned this to solution and
re-precipitated the double salt by directly adding solid K2SO4 instead of solution. This affords much, much cleaner
precipitation. Comparing the leftover solution from the original precipitation with the leftover solution from the second method, it's clear that
there's still some Nd left in the first one. So direct addition of the solid to the sulfate solution is definitely the way to go for effective and
complete separation.
[Edited on 6-11-2014 by MrHomeScientist]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
That's a good idea with the multiple anion mixture, but the only thing I've been using to precipitate the neodymium are sulfates, and the only other
anion in solution is nitrate - no hydroxides anywhere.
Also, I don't know if you've observed this, but whenever I heat the magnet nitrate solution, it turns very dark, but returns to its original color as
it cools down. Is this because the nitrates are decomposing in solution?
Just wondering, where would be the best place to store a magnet nitrate solution for easy transport? It's quite acidic at this point, and I don't want
to end my work now.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  |
Also, I don't know if you've observed this, but whenever I heat the magnet nitrate solution, it turns very dark, but returns to its original color as
it cools down. Is this because the nitrates are decomposing in solution?
|
One possible explanation is the following. Using nitric acid as the acid to dissolve the magnets, the iron is oxidised to Fe<sup>3+</sup>.
Ferric ion solutions are thermochromic because higher temperature pushes (simplified):
Fe<sup>3+</sup> + 2 H2O < === > Fe(OH)<sup>2+</sup> + H3O<sup>+</sup> to the right.
Fe(OH)<sup>2+</sup> is dark coloured in high concentrations (such as encountered in magnet soup). It appears this cation gives ferric
solution it colour ranging from yellow to orange to much darker colours because
[Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> appears to be almost colourless.
[Edited on 12-6-2014 by blogfast25]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | | That's a good idea with the multiple anion mixture, but the only thing I've been using to precipitate the neodymium are sulfates, and the only other
anion in solution is nitrate - no hydroxides anywhere. |
What I meant was that you have a 'magnet nitrate' solution, and are adding sulfate to this. I have a 'magnet sulfate' solution, and am adding sulfate.
So in my case there's more sulfate around, perhaps leading to easier precipitation. So my suggestion was to add hydroxide to precipitate everything,
then redissolve in sulfuric acid instead.
Although now that I say that, blogfast has been working with 'magnet chloride' with no problems... hm.
As for the reversible color change, I've definitely noticed that too. It looks like some sort of thermochromism, not related to the anion (since ours
are different). This experiment is full of surprises!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It shouldn't matter what the main anion in the soup was: the moment you saturate it with K2SO4, the least soluble crystallites, i.e. the (Ln(III),K)
double sulphates (as hydrates), crystallise out. Should also happen with nitrates present...
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I'm just spitballing here, but is it at all possible that a less soluble salt forms with the chloride along with the sulfate?
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | | I'm just spitballing here, but is it at all possible that a less soluble salt forms with the chloride along with the sulfate? |
It's not impossible but we have no evidence for it. Better to stick with what we know.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Back on the Wagon
Once again I've come back to this project - this time attempting the final step: lithium reduction of neodymium fluoride.
3Li + NdF3 == 3LiF + Nd
For the impatient, it appears to have been a failure. The hot lithium most likely reacted with the air. Read on the for details.
For this first test, I used my first batch of the fluoride since it is very obviously contaminated by iron. It's green under fluorescent light, and
pink in daylight.

After grinding to a fine powder, this was 1.4g NdF3. To this, I planned to react it with two slugs of my lithium, which was 0.17g.

Molten lithium is very corrosive to a wide variety of materials, so choosing an appropriate crucible has proven to be difficult. I went with graphite
this time, even though it is unsuitable for liquid lithium at 1000C (the target temperature here is 1024C, the melting point of Nd). I chose this
because, frankly, it was what I had. Also I figure the lithium won't be around for very long at that temperature anyway, having mostly been reacted
away by that time. Here's everything in the crucible.

I also did not use an inert atmosphere because, frankly, I haven't figured out the logistics of providing one to my crucible and furnace. I just
wanted to try it out, and see if it did anything at all. Shortly after starting heating, the lithium started glowing red hot. You can see by the
crucible that this is well under the temperature where this should be happening, so that must mean it reacted with something.

After heating for several more minutes with no change, I put the crucible in my mini furnace and cranked it up to somewhere in the neighborhood of
1000C. Nothing ever melted.

After cooling, I was left with a rock hard mass of grey crust. This stuff is nearly impossible to remove from the crucible.

No metal of any kind in sight. It looks like the lithium reacted with the air first. I guess I'll need an inert blanket after all. I figured as much,
but at least wanted to try this to see what happened.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Great stuff -- well kinda.
Watching you closely since I intend to do much the same with LaCl3
(after buying some fluoride, after converting to LaF3, after crystallising, after purchasing a crucible, maybe after lining it with Mo or Ta, after
building a furnace and after working out a way to feed argon into my setup...)
Right behind you.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
This is such an interesting project.
I'm probably missing something, but can't you cover the reaction in sand or something similar? Surely that's a lot easier than building an inert
atmosphere around it. Sure, you'll gets some silicon impurities from the lithium/SiO2 reaction but the molten Nd isn't going to reduce the sand is it?
And if the NdF3 is layered above the lithium, the sand shouldn't get in the way that much. You're going to have to physically remove it, but you'll
have to do that for the lithium slag anyway, even if a inert atmosphere is used.
If not sand, is there some other inert flux that will resist the high temperatures and the super reactive lithium? Aluminium oxide possibly??
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I had the same thought. But I was thinking excess NdF3. If the lithium was completely contained within a pile of the reactant then this would
resolve some of the containment and side reaction issues.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
@MrHomeScientist: don't you think that neodymium itself will burn in air at such high temperatures? If any of it was produced by reduction with
lithium, the product would look exactly the same (a mixture of lithium/neodymium oxide/fluoride).
Unfortunately, neodymium reacts with nitrogen when it burns (like lithium does). So if you can detect nitrides by reacting the product with water to
produce ammonia, it would not necessarily mean that lithium reacted with air.
[Edited on 27-10-2014 by Pok]
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
I recently made a small sample of neodymium sulfate by heating a magnet in concentrated sulfuric acid and then after letting the hot solution settle
decanting off the top layer, and then repeating.
Here is the end product:

|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Tdep: Molten lithium is some extremely corrosive stuff. We've been discussing it in a few other threads. The gist of it is that
pretty much any oxide is no good, because the Li will rip the oxygen out of it. It reacts with glass pretty vigorously, so sand would be a no go. I'd
definitely prefer inert blanket, if I can figure out how to do it.
Pok: Well the hope was that the LiF produced would form a protective layer over the denser Nd metal, if everything was good and
molten. I charged the crucible by putting some of the NdF3 on the bottom, then the Li ingots, then covered with the rest of the fluoride.
It might be better to put the Li on the bottom to better protect it and ensure the reaction goes from the bottom up, keeping the Nd buried and
protected.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Mr HS:
I think it could be useful to line the bottom of the crucible with a small excess of NdF3, to prevent the Nd metal sticking to the crucible.
Just an idea...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Perhaps... I'm torn between using an excess of lithium (precious and reactive!) and using an excess of NdF3 (takes literally years for me
to make!  ). ).
In my other experiences with graphite crucibles, the stuff just pops right out. So I don't know - could be indication of a reaction between C and Li?
I thought a bit more about inert atmosphere today, and this is my preliminary idea for such a setup:
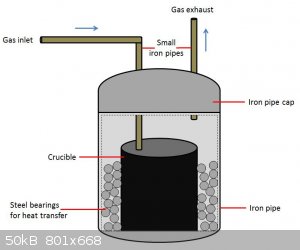
A crucible (iron, graphite, Ta-lined, whatever) is placed inside an iron pipe with space around it. This space is filled with small steel ball
bearings for heat transfer. An iron pipe cap is placed on top, with two holes drilled in it. Through these holes are inserted two smaller iron pipes
for gas entry/exit.
Argon flows in from the left tube, which extends down into the crucible. It exits from the right tube, which only just comes in past the pipe cap. The
entire assembly is heated from the outside via propane torch, and is expected to reach at minimum 1024C. (which is why copper won't work)
I have precisely zero experience working under these conditions, so if I have overlooked anything or if there is a severe safety hazard I'm missing
please let me know.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
MrHS:
A small excess of NdF3 helps also as follows:
NdF3 + 3 Li < === > Nd + 3 LiF
... is pushed to the right. But use an excess Li instead and where will it end up?
Re. your argon design, perhaps using copper wire instead of ball bearings? But if you're heating from the bottom then neither may actually do all that
much...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Well I wanted to avoid copper because the target temperature of Nd's melting point (1024C) is pretty close to copper's melting point (1085C). The last
thing I want is a non-nuclear meltdown!
It might not be clear from picture 5, but my furnace has the torch come in from the side. Similar to picture 4. I figure I'll need to heat the whole
thing up to 1024 to ensure molten products. I don't know if the reaction itself will proceed with heat to spare ala thermite.
Come to think of it, now that I have this furnace setup, is magnesium reduction a possibility again? We scrapped that because it didn't produce enough
heat of reaction, but if I heat the whole thing externally that won't be a problem. Then I wouldn't need inert atmosphere either, which would simplify
greatly. In fact I think I still have the Mg + NdF3 mixture that failed from my first attempt...
|
|
|
| Pages:
1
..
14
15
16
17
18
..
24 |