| Pages:
1
..
13
14
15
16
17
..
22 |
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks j_sum1. Yeah, I'm back... now with 20% more danger and less referencing 
Yes, it does seem too good to be true, but that said, melamine definitely reacts even when very diluted in water. It might just be that melamine is
'special' in this regard.
/speculation
I have a sneaky suspicion that the melamine-hypochlorite oxidation proceeds via formation of cationic amine oxide radical on the melamine's ring
nitrogen position (stabilised by delocalisation), hence the extreme bright red colour and very high solubility of that intermediate (thinking of TEMPO here). i.e. something like this:
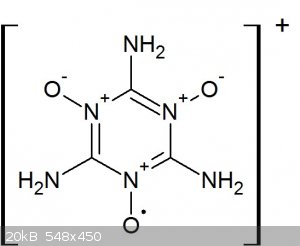
I think when you add more oxygen to that molecule, it simply can't hold itself together anymore and starts to break up with the fragments oxidising
fully.
/speculation off
So yeah, while urea's carbamate nitrogen groups would probably be far more inert, I think it's worth a shot since I did get the melamine-hypochlorite
oxidation to work.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Where do you get melanine ?
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Darker skin and brown eyes mostly.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Melamine chemical is different from melamine the popular name for the polymer although the two are related.
Melanin the pigmentation is completely unrelated. It is an etymological fluke that the two are only a couple of letters apart.
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Quote: Originally posted by j_sum1  | Melamine chemical is different from melamine the popular name for the polymer although the two are related.
Melanin the pigmentation is completely unrelated. It is an etymological fluke that the two are only a couple of letters apart.
|
Wait, in english the pigment is called melanin!? Dang it! Completely messed up 
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Hey. I'm no expert. I just looked it up.
https://en.wikipedia.org/wiki/Melamine#Etymology
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Exactly so, melamine (the resin plastic that is used to make dishes) is made by reacting melamine powder (the chemical) with formaldehyde. This makes
a very hard resin similar to Bakelite, except that instead of phenol units linked by methylene bridges, it's now melamine units linked by methylene
bridges.
Melamine powder (the chemical) is not OTC as far as I know. I bought mine from a chemical supplier a very long time ago (more than ten years). I don't
have any left, sadly 
I did tons of experiments with my melamine powder and it's a pretty interesting chemical. For example, it forms nearly completely insoluble salts with
virtually all mineral acids INCLUDING nitric acid! I don't know any other insoluble nitrate salt except for melamine nitrate, but I'm sure SM members
will be able to name some 
If we get desperate (i.e. urea is not oxidizable by hypochlorite), then one possibility is to grind/file up a cheap melamine plate into a powder and
try oxidizing that  The extra methylene units will, however, consume much more
hypochlorite per amount of NO2 you would make, which would be wasteful. I hope it won't come to such desperation! The extra methylene units will, however, consume much more
hypochlorite per amount of NO2 you would make, which would be wasteful. I hope it won't come to such desperation!
j_sum, when you scope the initial test-tube amounts for the urea-hypochlorite reaction, if you get nothing initially, try gentle
heating. I really don't want to have to file plates, besides, melamine resin might be too inert to react with hypochlorite.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
A cursory search of urea-hypochlorite is yielding surprising results. Many sites mention the formation of explosive nitrogen trichloride, which was
unexpected as NCl3 requires acidic chlorinating conditions to form AFAIK.
It may be that the CO2 formed during reaction drops the pH by precipitating CaCO3.
While commercial calcium hypochlorite may contain lime as an impurity, very pure calcium hypochlorite might become more acidic as the reaction
proceeds.
I strongly advise adding some lime to the material to ensure basic conditions and so minimise the risk of forming explosive NCl3 when
working with very pure calcium hypochlorite.
It would also capture more of the CO2, thus decreasing evolved gas volumes.
The hypothetical equation would then be:
7Ca(ClO)2 + 2Ca(OH)2 + 2(NH2)2CO => 2CaCO3 + 7CaCl2 + 4NO2 + 6H2O
That said, having NO2 evolve under basic conditions is another suspect matter  This reaction is proving more complex that I would have thought or liked
This reaction is proving more complex that I would have thought or liked  
If you calculate what the mass percentage of calcium hypochlorite is in the calcium hypochlorite + calcium hydroxide stoichiometry indicated above, it
comes to 87%.
Thus pool 'HTH type' granular chlorine, for example, with specified 68% hypochlorite, may in fact already contain enough lime, assuming the residual
mass is a mixture of calcium hydroxide and chloride.
This is what I was using with my melamine chemistry and may be why I didn't observe any formation of NCl3.
[Edited on 27-6-2015 by deltaH]
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
I've been this close from trying to do thermal oxidation of urea due to the utter nothingness I found when looking for it online....
then it occurred that I don't have a fume hood and I'd probably be safer asking around here.
So, anyone have any idea on what I should expect to form from this sort of reaction? With the ease of getting refractory bricks it shouldn't be that
hard to build a thermal oxidation reactor... heck, maybe some transition metal catalyst like those used in oxidation of other organic compounds could
be used to lower the heat necessary.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I don't see urea forming NOx by thermal pyrolysis because of the pathway that urea pyrolysis follows 
There is a good thread on thermal decomposition of urea on here somewhere, use google's site search. There's also a nifty review paper on the topic on
"cyanurates" that will give you ABSOLUTELY everything you want to know about it. If you don't find it, PM me.
Basically if you mix the appropriate amount of slaked lime and urea and heat, you will first produce copious amounts of ammonia  and ultimately if you get to red heat, calcium cyanamide with the evolution of lots
of CO2. Note: Without the lime, you will release toxic isocyanic acid in large amounts! and ultimately if you get to red heat, calcium cyanamide with the evolution of lots
of CO2. Note: Without the lime, you will release toxic isocyanic acid in large amounts!
The calcium cyanamide can be converted to melamine in theory (by acidification with a calcium precipitating weak acid, eg. CO2 and boiling down the
resulting cyanamide solution after filtering off the carbonate) though all in all, this is no trivial matter.
As I see it at the moment, there's two possibilities with urea:
(1) The hypothetical low-temperature oxidation with some super oxidant.
(2) Making ammonia from it and then catalytically oxidising the ammonia at high temperature with oxygen.
The second option is tough to execute, hence only option (1) seems plausible in practical terms, though whether the chemistry will play along is
[highly] suspect at this stage.
[Edited on 28-6-2015 by deltaH]
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Quote: Originally posted by deltaH  | I don't see urea forming NOx by thermal pyrolysis because of the pathway that urea pyrolysis follows 
There is a good thread on thermal decomposition of urea on here somewhere, use google's site search. There's also a nifty review paper on the topic on
"cyanurates" that will give you ABSOLUTELY everything you want to know about it. If you don't find it, PM me.
Basically if you mix the appropriate amount of slaked lime and urea and heat, you will first produce copious amounts of ammonia  and ultimately if you get to red heat, calcium cyanamide with the evolution of lots
of CO2. Note: Without the lime, you will release toxic isocyanic acid in large amounts! and ultimately if you get to red heat, calcium cyanamide with the evolution of lots
of CO2. Note: Without the lime, you will release toxic isocyanic acid in large amounts!
The calcium cyanamide can be converted to melamine in theory (by acidification with a calcium precipitating weak acid, eg. CO2 and boiling down the
resulting cyanamide solution after filtering off the carbonate) though all in all, this is no trivial matter.
As I see it at the moment, there's two possibilities with urea:
(1) The hypothetical low-temperature oxidation with some super oxidant.
(2) Making ammonia from it and then catalytically oxidising the ammonia at high temperature with oxygen.
The second option is tough to execute, hence only option (1) seems plausible in practical terms, though whether the chemistry will play along is
[highly] suspect at this stage.
[Edited on 28-6-2015 by deltaH] |
Well, I'm really glad I decided to ask 
Good readyng material from the urea pyrolysis topic, thanks for the heads up. Couldn't find the cyanurates paper but its okay since I don't have my
proxy set up for getting access to RSC and others anyway.
I may start reading on catalytic oxidation of ammonia now that my dreams of oxidating urea have been crushed, but I really can't imagine a easily
built reactor in this case since handling gases never worked well for me.... when I thought of the urea decomposition I had in mind a batch reactor
that could be sealed with the solid compound inside and left to react without bothering with feed streams and stuff.... But oh well, its never that
easy is it? 
Lastly it probably should've occurred to me that pyrolysis and thermal oxidation were the obvious search keyword, not oxidation.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Following deltaH's suggestion, I performed a couple of tests on urea and calcium hypochlorite. Here are the results.
Small scale: 0.1g urea, 0.13g Ca(OH)2 and 0.83g Ca(OCl)2 Drop of water to initiate.
A reasonable amount of gas evolution. Wishful thinking may have labelled it as brown.
Larger scale: 1.0g urea, 1.33g Ca(OH)2 and 8.33g Ca(OCl)2. Gas fed into H2O2. Drops of water via sep funnel to get the reaction going and speed it up
if/when necessary.
Gas evolution began at low rate before water was added. At the first drop of water large volume of gas was produced. Lots of white fumes filled the
flask and came through the apparatus. It was smokey in appearance and my guess is that it was particulate CaCO3. Reaction was very exothermic and
flask became hot to touch.
System experienced suck-back which meant that I lost the H2O2. I added some more and continued to attempt to dissolve the gas. Again a wishful
thought might claim a brownish tinge but it definitely was not pure NO2.
Solution at the end still contained a lot of peroxide (unsurprisingly). It was a very light brown colour. pH was around with indicator paper. No
visible reaction between this and copper wire so I guess that HNO3 content is minimal.
Reaction with the Ca(OH)2: 0.2g urea, 1.7g Ca(OCl)2
Reaction began while powders were being stirred. Quite loud explosive pops which presumably came from NCl3. Rather an exciting experiment but
ultimately a bit fruitless. I don't think I would want to upscale that at all. at 0.2g scale it was quite percussive on the glass beaker I was
using.
So, interesting findings ,but probably no progress made towards the HNO3 goal.
Still, good to eliminate one possibility.
[Edit]
Adding photos
(1) Apparatus setup
(2) Gas evolution (after it had tamed a bit. I think the particulate suspension is CaCO3.
(3) Slight discoloration of the liquid but nothing remarkable
(4) No effect on copper wire
(5) But clearly acidic. Is this carboxylic acid?
Now the mission is to figure out what reaction has actually occurred.
[Edited on 30-6-2015 by j_sum1]
    
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks for experimenting j_sum1, I really appreciate the effort!
I'm glad my suggestion of lime was not a pointless precaution, in a moment of weakness, I thought I might have been silly suggesting it 
You might be forming calcium nitrate in solution because of the necessary basic conditions.
Another idea is to swop the order of addition and try to target the formation of NO (nitric oxide) gas instead to avoid forming nitrates. This is then
easily oxidised externally to NO2 gas.
e.g. Prepare a saturated urea solution and add the lime-hypochlorite incrementally/gradually --- weaker oxidising conditions targeting NO gas
formation that should bubble out due to low solubility.
The reaction stoichiometry would change of course, but on the upside, you can economise on hypochlorite!
[Edited on 30-6-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
You keep editing your post deltaH! 
All good suggestions. I see merit in adding a solution to the solid. If nothing else it saves me the task of grinding both the urea prills and the
hypochlorite. Before the next round however, I think I want to be a bit clearer on what the reaction products have been. There are obviously a few
possibilities.
As for conserving Ca(OCl)2, it is the least of my worries. I have a couple of kilograms and more is available from the supermarket less than 300
metres from my house.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Ha ha, yes I'm notorious for that, trying to decide what are good suggestions and then keep coming up with better trains of thought  , so this is MkIV version lol , so this is MkIV version lol
Very nice photos and work, thanks again!
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I like the one with the fuming test tube. I might have to do that one again just for the fun of it.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I'm sorry guys, but if I remember correctly in the old hydrazine thread this reaction (hypochlorite + urea in a big excess) was utilized to synthesize
hydrazine hydrate, why do you thing you'll get other things out of it?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by papaya  | | I'm sorry guys, but if I remember correctly in the old hydrazine thread this reaction (hypochlorite + urea in a big excess) was utilized to synthesize
hydrazine hydrate, why do you thing you'll get other things out of it? |
Because I don't know any better?
Seriously, playing with urea is new ground for me. I know next to nothing about it. I will have to look that one up.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Because hydrazine is a reducing agent and there's excess very active oxidant? Not saying it's not forming, right now we haven't yet pinned down
exactly what is forming in the oxidation 
My big worry is that it's making N2 gas (and CO2). That would be rather useless aside for airbag generators, lol.
The oxidising conditions are very aggressive with calcium hypochlorite, but maybe hydrazine is forming in situ and then oxidising to N2?...
which would be really bad 
Way back, when mucking about with melamine, I found that sodium hypochlorite didn't oxidise melamine, but calcium hypochlorite did. So there's a
difference there too, maybe.
Your first picture is very  j_sum1 j_sum1
Maybe try making some coloured hydrazone's with your solutions to see if there's hydrazine in there? Do you have almond essence (not even sure if that
makes a yellow hydrazone). In a test-tube, acidify with a few drops acid, add benzaldehyde solution and heat?
[Edited on 30-6-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Okay, so you probably want more proof that me 'just saying' that hydrazine shouldn't survive hypochlorite excesses. 
There's a report from the US military at this link:
http://www.dtic.mil/dtic/tr/fulltext/u2/a213557.pdf
where they tested hypochlorites to decontaminate hydrazine spills. They found that the reaction of both liquid bleach and solid bleach (calcium
hypochlorite?) on dilute hydrazine solutions was "rapid", highly exothermic and evolved almost entirely nitrogen gas for unsubstituted hydrazine.
So as I see it, if hydrazine was produced, then it would decompose very quickly to N2 in that reaction. If N2 is the main gas produced, then this is a
possible reaction pathway: -NH2 => ClNH2 => NH2NH2 => N2
Else calcium nitrate may have formed if the gas evolved was not nitrogen (and carbon dioxide).
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Yes, oxidation of hydrazine in most cases yields nitrogen gas, sometimes some amount of azide can be formed, which can also further be oxidized to
nitrogen. IMHO what you should look for is a method (maybe some well known in organic synthesis) to oxidize amine (or amide) group to a nitro one,
then maybe you have a chance to "extract" that in form of NO2 gas or nitrate.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I fear that I have somewhat hijacked this thread and that my current pursuits are only tangentially related to the goal in mind (and are unlikely to
get closer.)
With that disclaimer aside, I should report back on the calcium hypochlorite and urea adventures.
It seems that water is more than an inert reaction medium in this case. I added saturated Ca(OCl)2 solution to urea prills to observe what would
happen. No Ca(OH)2 used this time.
Immediate fizzing of the prills. Definite smell to the gas given off. Sort of chlorine-y but less sharp. Similar to the
smell of most pool chemicals I guess, but maybe with a hint of ammonia to it. I am prepared to concede that there may be more than one gaseous
species evolved.
No popping characteristic of NCl3 this time. Even though after a while I worked up to greater quantities than last time.
Contents of the reaction vessel appeared a light pink colour.
Wet indicator paper placed in the gas stream did not change colour. Well, not quite true. After some time the paper dried a bit and appeared
to bleach a bit too.
I have to conclude that the presence of water changes the reaction that proceeds. I have really little clue as to the nature of the products.
The next thing to do is to attempt the reaction in dry conditions with the inclusion of Ca(OH)2. I might try some excess of the lime as a CO2 mop. I
am going to predict minimal NO2 production if any. I will report back when done.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Very interesting j_sum1.
| Quote: | | Immediate fizzing of the prills. Definite smell to the gas given off. Sort of chlorine-y but less sharp. Similar to the smell of most pool chemicals I
guess, but maybe with a hint of ammonia to it. I am prepared to concede that there may be more than one gaseous species evolved.
|
Sounds like chloramine. Depending on the pH, you could be making chloramine, dichloramine and finally trichloramine the more acid the reaction gets.
It also sounds like the oxidation is too mild in solution to go beyond the 'standard' chlorinations of ammonia.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2786
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
In the presence of Br-, O3 takes NH3 >> NO3-:
http://www.sciencedirect.com/science/article/pii/00431354849...
Without Br-, O3 takes NH3 >> NO3- only at high pH. This reaction is mostly studied in wastewater treatment and has never been optimized to
produce nitrate. However Br- is easy for us to come by and the mechanism sounds both plausible and likely to generate the right product (O3 reacts
readily with NH2Br, it seems).
Ozonation is questionably OTC. Ozone is a toxic gas, after all. However its production is so well-studied there is even a tutorial reference for an
undergraduate lab assignment, where sulfuric acid is electrolyzed:
http://pubs.acs.org/doi/abs/10.1021/ed082p1546
http://jes.ecsdl.org/content/129/3/506 (a comparison of electrolytic O3 generation methods)
And of course ozone generators are a well-known thing.
| Quote: | | I performed a couple of tests on urea and calcium hypochlorite. |
I'm sorry, but I really don't think urea is going to work. An amide like urea is delocalized across the N-C-O bond system which forces the nitrogen
into a planar configuration. Oxidations of amines usually proceed through tetrahedral intermediates, whereas amides undergo a reaction more like
electrophilic aromatic substitution (amides react with TCCA or NBS but amines react with KMnO4 or K2CrO3) which usually produces a halo-amide. That
rearranges to hydrazine, and once you're at hydrazine it's just too easy for an oxidation to produce N2 instead of the desired product. Best thing you
can do with urea is probably to hydrolyse it.
[Edited on 1-7-2015 by clearly_not_atara]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Interesting what you said about the configurations clearly_not_atara.
Ozone sounds like fun, but not the OTCishness we need.
But that got me thinking, if that works, maybe singlet oxygen might as well? That can be prepared from a hypochlorite and hydrogen peroxide according
to Wikipedia, so it's OTC for some of us. If I understand you correctly, a bromide salt in there may act as a catalyst?
So I guess what I'm proposing is 'upgrading' the urea-hypochlorite reaction by adding hydrogen peroxide and a bromide salt, say KBr as catalyst? Is it
really necessary to have bromine in the fray?
Hypothetical equation:
14H2O2(dil)+7Ca(ClO)2(s) + 4Ca(OH)2(s) + 4(NH2)2CO(aq) =>[Br-(cat)]=> 4CaCO3(s) + 7CaCl2(aq) + 8NO2(g) + 26H2O(l)
Exactly how one is supposed to excecute such an addition without blowing yourself up ... 
Simultaneous dropwise addition of two solutions (H2O2 and separately, urea) to hypochlorite prills with some lime in a flask?   The timing
is going to be really hard to get right because of the very fast reaction of urea and hypochlorite alone. What a mess The timing
is going to be really hard to get right because of the very fast reaction of urea and hypochlorite alone. What a mess 
Could be easier on a test tube scale with two pipettes and a simultaneous squart of a ml with a tiny amount of hypochlorite and lime in the bottom of
the tube?
PS. Singlet oxygen is toxic AFAIK, be forewarned 
[Edited on 1-7-2015 by deltaH]
|
|
|
| Pages:
1
..
13
14
15
16
17
..
22 |