| Pages:
1
..
12
13
14
15
16
..
20 |
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
First of all, I would strongly encourage anyone who wants to work with alcohol dehydrogenation, or catalysis in general, to read the Google Books link
that I posted at the end of my previous post. The book was written by Sabatier, and I think of him as the grandfather of catalysis. The book is free
to download. I lot of what I have learned, I learned from this book.
The decomposition has more to do with catalyst activity than bed size. Copper is the active catalytic material, and its activity varies according to
how the catalyst is prepared. Copper hydroxide, gently reduced at the lowest possible temperature (250-300C), will take on a dark violet coloration.
This is a very active form of copper, as it has very high surface area. It works well for ethanol dehydrogenation, but is too aggressive for
methanol. Copper hydroxide that is dead-burned (as the oxide) at prolonged read heat will have much less surface area. It is too sluggish for
ethanol, but works OK for formaldehyde production. Upon reduction it will usually have a bright copper color.
Quote: Originally posted by Oxirane  |
I understand the linked paper as following:
- First in solution are dissolved nitrates of Mg, Ca, Sr and Cu, then mixed in Silica gel, then stirred, filtered, dried and calcined.
- Then nitrate of barium is dissolved ontop of the all previous, and stirred, filtered and finally calcined all over.
-Then the catalyst is flushed with hydrogen for 2 hours at 300C to produce active catalyst.
How can calcium, strontium and other be calcined at 450C from their nitrates while their decomp temperatures are way over 600C? Both temps are
somewhat easy to achieve with normal gas burner with insulated container, but that would bring some low-decomposing oxides, like copper, into such
high temperature, could we be afraid of heating them "dead", as some mentioned CaO can be done?
|
Nitrates are strong oxidizers, especially when heated to 450C. In the presence of reducing agents they won't last long at high temperatures.
I haven't tried the exact procedures outlined in that journal article. It's possible that the silica support helps the catalyst maintain activity
even after heating to high temperatures. Fine copper powder (as mentioned above) actually works fine for dehydrogenation. The main reason that other
elements are used is to minimize catalyst deactivation. Prolonged heating of fine copper in a reducing environment will eventually sinter the copper
into a low surface area material. This may take several hours, and may not be a problem for non-industrial processes. I'm using copper/magnesium
oxides in my tests. The magnesium oxide helps the catalyst maintain activity over a longer period of time that copper alone. As I mentioned before,
I haven't re-found the exact reference that I pulled this idea from. It was a journal article.
Quote: Originally posted by Oxirane  |
Is the strontium necessary, or should it work with Ca+Cu+Mg+Ba only?
How should the catalyst be protected from air, or should it be prepared in situ for each dehydrogenation reaction? Once the cats are applied on the
SiO2 substrate, it don't have to be done again anymore, but most likely all the metals will be coated by an oxide layer the second they meet the
atmosphere.
Should one use pure copper only for formaldehyde dehydrogenation if the reactivity is too large for previous metals?
It has been demonstrated that pure copper tube heated to dull red was capable of dehydrogenating ethanol into acetaldehyde in Versuchschemie forum.
They got from several hundred ml's of ethanol a 100ml of acetaldehyde. They never tested how long would the catalyst live.
|
Just prepare the catalyst the way that I did earlier, and that should be an easy start. The catalyst doesn't need to be protected from air. It will
oxidize, but it will immediately be reduced again once alcohol vapor is passed over it. I never use hydrogen to reduce the catalyst. Alcohol vapors
work just as well.
For methanol dehydrogenation, copper pipe may work as long as it has been oxidized and reduced a few times on the inside. It may take considerably
higher temperatures with that type of catalyst, though. Read through the book for more explanation on this.
|
|
|
Oxirane
Hazard to Self
 
Posts: 92
Registered: 19-9-2014
Member Is Offline
Mood: No Mood
|
|
Brilliant, thanks for the information. I will have to reek through the topic with time and read the documents as well.
Could the impregnation of silica gel be done in such way that copper and magnesium sulfates are dissolved in water, silica gel is added and stirred so
they soak up, and then sodium hydroxide is introduced until no more precipitation occurs, and then filtering, washing and decanting the silica gel?
This would save the need for preparing water soluble nitrates. Calcium chloride could also be added, since this co-precipitates as a calcium
hydroxide. This would need the heat to be brougt at 580C at calcination stage in order to decompose the water form, but how important calcium oxide
promotor will be, is another matter. Calcium hydroxide is also quite water soluble in relation to catalytic amounts deposited, so upon washing the
silica gel with small amounts of water could dissolve most of the deposited Ca off.
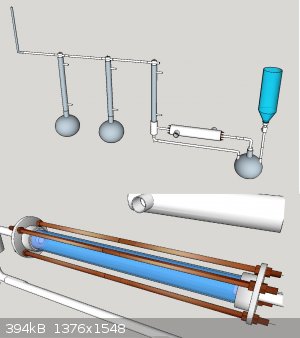
I am planning to use the apparatus I presented in acetanhydride topic. The setup is somewhat general and the operating principle is simple. The
reactor could be placed vertically as well, but this way the catalyst will hold in there easier.
Unreacted methanol is always more preferable than decomposition, since methanol can be recirculated indefinitely through the bed with refluxing
recirculation, but CO and H2 are total loss.
I don't believe 300C will sinter metallic copper in any reasonable time. Other tale is when using gas torch which can put 800-1200C surface temps.
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Sodium hydroxide reacts with silica gel to some extent, forming silicates. Otherwise, you'll have to try it and see.
Pure copper is very soft. Unoxidized nano-copper can be smeared into a copper streak at room temperature. At higher temperatures this occurs more
readily. When milling copper, I've seen the warm copper chips immediately weld themselves to the work-piece while I cut. This is caused by a
combination of warmth, an extremely clean surface, and pressure. Gold does the same thing. It is so soft and inert that it can be hammered into one
piece without heating. According to the book, 300C is about the highest temperature that one wants to go with a pure copper catalyst, as the copper
rapidly loses surface area above this temperature.
[Edited on 11-7-2014 by WGTR]
|
|
|
Oxirane
Hazard to Self
 
Posts: 92
Registered: 19-9-2014
Member Is Offline
Mood: No Mood
|
|
That only shows that "I think" is not worth of "I know". It should be taken into consideration, then. The germans got, as far as I know, acetaldehyde
made from ethanol by just heating copper tube without any packing, so even some copper wool would increase the surface area by thousands of times.

I need to do some tests on that silica gel-hydroxide subject. If silica gel absorbs sodium hydroxide, or copper sulfate is absorbed inside and
reaction is incomplete, there may be some side-effects. Copper sulfate can be pyrolyzed at over 600C, but that's rather high temperature. This was my
first plan because of its straightforwardness.
And I obviously have to assemble the reactor too. 
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
I've thought of a potentially OTC method that I haven't seen mentioned yet.
Dehydrohalogenation of dichloroethane to vinyl chloride gas followed by nucleophilic substition to vinyl alcohol which rearanges to acetaldehyde.
1. C2H4Cl2(l) + NaOH(al) -> C2H3Cl(g) + NaCl + H2O
2. C2H3Cl(g) + NaOH(aq) -> C2H3OH + NaCl
3. C2H3OH -> CH3CO
This method only uses dichloroethane (a fairly common solvent and easily made from ethylene glycol), sodium hydroxide and an alcoholic solvent. It
also can be done with fairly basic glassware.
The main drawback I can see is the highly toxic nature of vinyl chloride gas. Thoughts?
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi folks, I custom made a new alcohol oxidation reactor, since I broke the old one (I almost cried).
I will also try to oxidize alkanes with it, C3-C6 to see what I can get, and how far the oxidation goes, hopefully not till H2O+CO2. Note that the
kanthal filament will be inside the glass tube, instead of heating the glass wall, similar to a ketene lamp.

|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
That's some nice glassware. I'm jealous!
I had always wondered what happened to you. I had followed your experiments in this thread back in the day, and tried some of them myself. I hadn't
noticed your posts in the last year.
As an FYI, Teflon-insulated stranded wire uses silver-plated copper strands. I took an individual thin strand, wound it into a double-helix, and
found that it didn't take too much current to get it to heat up.
I have personally gravitated towards using a tube furnace configuration with catalyst beads, since that is easy for me to build. What I have never
done very well is the acetaldehyde condensation and separation from the excess alcohol. I haven't put the necessary time into fine tuning this
process.
So, is the reactor intended to work horizontally? And, did you make it yourself?
[Edited on 3-15-2015 by WGTR]
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi WGTR tube furnace are the way to go, I had trouble finding someone who would forge me NS-quartz tube end joints made of ceramics or graphite, so I
decided to go with glass. I have it prepared with a glass blower for 30dollars + thermometer for 8dollars.
When I made acetaldehyde with catalytic dehydrogenation. I never let O2 into the system. Just let it boil and react with copper wire, the overhead
condenser was on max cooling capacity i.e. below the boiling point of acetaldehyde @ around 5-10°C. At this temp the acetaldehyde slowly makes it
into a trap kept at -30°C. This way I could make some 20-30ml pure acetaldehyde at each 8hrs run. I send a sample of it once to a friend who measured
it with HSGC-MS and said it is of lab grade purity.
Of course you can let air into the reaction zone to speed the process but I think you would only complicate things.
The reactor will work vertically the uncondensed liquid will flow back to the reboiler.
Catalysts in mind:
Standard copper oxide made with anodic oxidation of copper metal.
Silver oxide on ceramic rings.
Chunks of catalysts from a catalytic converter, from diesel ang gasoline car too. (diesel has too much platinum and it favors the oxidations to go way
too far, but we will see)
Copper oxide on graphit, pressed into granules (from some industrial oxidation reactor)
I had to depart from chemistry for family reasons, but I am back, so to speak
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Here is the reactor in operation, the kanthal wire was brought to dull red, the gas evolution was continuous. I led the gas into ccNaOH sol, after
~10mins and orage precipitate appeared with an intense apple smell. The gas was led into 1ml of ccH2SO4 after ~15mins it charred. I did not try to
isolate the acetaldehyde since it would require some -30°C temps.
  
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's an interesting looking apparatus, Jimmy. What is the side tap with the stopcock for?
I haven't read this thread but I'm wondering if forming a bisulfite adduct of the acetaldehyde in situ would be a good way to isolate it.
Edit: I skimmed through to page 9 where I found your link to Len1's method for benzaldehyde where he purifies with the bisulfite adduct. I don't
know how this would work out for acetaldehyde, however.
[Edited on 16-3-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi Magpie, the tap is for letting air in below or above the filament, lack of good mixing of the vapors may cause a mini explosion, that is why I
still collecting some nerve to try it.
Another method would be to electroplate copper onto the kanthal and heat up the wire under liquid Et-OH itself. It would be interesting to see what
happens, maybe all the heat would go into acetaldehyde synth insted of refluxing the Et-OH.
The setup for this is as simple as this:

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm really not up to speed on this thread or any reading on making acetaldehyde but I have made/attempted to make propionaldehyde and butraldehyde by
oxidizing the alcohols per Brewster. My yields were poor or non-existent.
Why do you have to introduce air? Doesn't the ethanol supply the oxygen for the -CHO? I like your simplified apparatus, though.
CH3-CH2-OH -----> CH3CH=O +H2
My thinking on the bisulfite adduct was to simply place sodium metabisulfite in the pot and reflux away, condensing everything back into the pot.
Hopefully the acetaldehyde would form the adduct which might be a filterable solid in ethanol. But if the bisulfite is not soluble in ethanol then
this likely would not work.
[Edited on 17-3-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by Magpie  | | My thinking on the bisulfite adduct was to simply place sodium metabisulfite in the pot and reflux away, condensing everything back into the pot.
Hopefully the acetaldehyde would form the adduct which might be a filterable solid in ethanol. But if the bisulfite is not soluble in ethanol then
this likely would not work. [Edited on 17-3-2015 by Magpie] |
That's an interesting idea. I can't say that I ever thought of that one before. A previous post by Nicodem may answer your
question:
http://www.sciencemadness.org/talk/viewthread.php?tid=6118&a...
I don't know if steam would have any affect on alcohol dehydrogenation. It may actually help it, by diluting hydrogen in the reaction products, and
preventing alcohol dehydration. I don't think dehydration is a significant problem with copper catalysts, though.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for that good information WGTR. This gives some hope for the method and may justify some experimentation. I see another potential problem,
however: assuming that the adduct can be made and removed by filtration now it must be reacted with NaOH to recover the aldehyde. Without reading to
find out why I understand that this harms the aldehyde. Cannizzaro reaction perhaps? However, I know that other aldehydes are recovered this way.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
IIRC the bisulfite should be used in large excess since it is a reversible reaction.
What about reacting the aldehyde with ammonia wapor in ethyl acetate? When the reaction is done. Cool the slurry and filter the crystals.
Magpie:
Et-OH <=> CH3CHO + H2 it is a reversible reaction, you have to eliminate the H2 to speed things up. Air is a cheap reagent for this purpose
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Jimmymajesty  |
Magpie:
Et-OH <=> CH3CHO + H2 it is a reversible reaction, you have to eliminate the H2 to speed things up. Air is a cheap reagent for this purpose |
Does the H2 react with oxygen  or is the air just a purge gas? or is the air just a purge gas?
If just a purge gas replace it with N2 or argon.
---------------------------------------------------------------
I just came back from the lab where I tested the solubility of K bisulfite in absolute ethanol. I placed 1g of the salt in a small flask and then
added 5 ml of abs ethanol, then heated to boiling. There was no dissolution. I then added distilled water, 1 ml at a time, then bringing to a boil.
It took the addition of 12mL of water before the salt was completely dissolved. I will now repeat this experiment with Na bisulfite (if I can find it
in my cabinet) and report back.
--------------------------------------------
Remarkably, under the same conditions, the Na bisulfite only required the addition of 3 ml of water to effect complete dissolution at boiling. I will
now attempt to find out how much Na bisulfite, if any I can dissolve in 5ml of abs ethanol, and report back.
-------------------------------------------
Even with boiling and stirring I could not get even 0.13g of Na metabisulfite to dissolve in 5ml of absolute ethanol.
[Edited on 17-3-2015 by Magpie]
[Edited on 17-3-2015 by Magpie]
[Edited on 17-3-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Magpie, air is to remove the H2 (oxidize) from the surface of the catalyst.
Today my curiosity conquered my fear and let air into the reactor through the upper tap, the flow was around 1ml/sec but nothing really happened. The
temp on the thermometer was remained 110°C, the gas evolution was about the same, maybe it was not enough to make any change, I will give it another
go in the coming days.
This time I went with platinum (+rhodium) catalyst scavenged from a catalytic converter of a diesel car.
The thermometer with this type of catalyst was not read as much as with copper, but what does it tell you? Is the platinum cata better because the
reaction without air is slightly endothermic?
 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Jimmy, I did a little more reading of this thread and now realize how much work you have done. You probably know more about this synthesis than
anyone else on this forum.
I did read in Gattermann (1937) in the forum library that making acetaldehyde-ammonia, a solid, is a practical way to store it, as is making
metaldehyde. His procedure looks quite long and complicated.
I really have no plans for making it, although I would like to have some for making crotonaldehyde.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Magpie, any luck with the bisulphite method?
I did a two hours run and collected ~20ml, it started to boil by simply holding the glass in hand. Maybe let the vapor onto dry ice is the only option
since the refrigerator was ~-10°C still the acetaldehyde smell was as pungent as without the fridge.
I also did a run with methanol, there was not any formaldehyde present. I realized when I came home that there should not be any, since the CH3-OH
requires oxigen surplus to be oxidized and on Pt it tends to go till H2O+CO2.
I hoped to be able to co-oxydize Me-OH with Et-OH to get their aldehydes in the same mixture, but you would need 1-2gMe-OH in 2litres of air to get
the oxidation going.
Anyway the Me-OH is a good hydrogenating reagent in this setup (CH3-OH => CO + 2H2) and I think with copper it could reduce many things like NO2
groups to NH2
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice going Jimmy. You may be the only person on this forum to successfully make acetaldehyde in any significant quantity. Well done.
Did you miss my report from an earlier post? See below.
Quote: Originally posted by Magpie  |
---------------------------------------------------------------
I just came back from the lab where I tested the solubility of K bisulfite in absolute ethanol. I placed 1g of the salt in a small flask and then
added 5 ml of abs ethanol, then heated to boiling. There was no dissolution. I then added distilled water, 1 ml at a time, then bringing to a boil.
It took the addition of 12mL of water before the salt was completely dissolved. I will now repeat this experiment with Na bisulfite (if I can find it
in my cabinet) and report back.
--------------------------------------------
Remarkably, under the same conditions, the Na bisulfite only required the addition of 3 ml of water to effect complete dissolution at boiling. I will
now attempt to find out how much Na bisulfite, if any I can dissolve in 5ml of abs ethanol, and report back.
-------------------------------------------
Even with boiling and stirring I could not get even 0.13g of Na metabisulfite to dissolve in 5ml of absolute ethanol.
|
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hi Magpie, I would have not even considered making it if sciencemadness would not have made it seem easy
I read your post but what about the adduct? Could you isolate any aldehyde with it?
I did some test with ethanol today paving the road to formaldehyde synth, it may be obvious but it give me a lot of idea what should/shouldn't be done
with methanol.
The setup was rigged to be able to let air bubble through ethanol and there was a kanthal filament under the liquid to regulate its temp.
Findings:
If the methanol at room temperature no matter how much air you pump through it it will not ignite
If it is warm and you increase the air flow there is always a point when an explosion occur.
If the air flow is constant and the liquid is constantly heated it starts to boil, when the condensation front reach the ignition source it starts to
burn without explosion, at this state no matter how much air I pumped through the boiling liquid I could not make an explosion.
So basically you can do anything with the vapors, they are way above the explosion limit of the mixture, of course it is only true for this test
device so be careful with your own setup.

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes! There is no limit to what we can do. It's only a matter of time.
I'm presuming that since ethanol is the feedstock for making acetaldehyde, and I could not get the bisulfite to dissolve in ethanol, it would be of no
use for your synthesis. But once you make the acetaldehyde I would certainly think that you can add it to the product mixture and form the aldehyde
adduct, a solid. I have used it this way before, successfully. It's a commonly used technique.
Quote: Originally posted by Jimmymajesty  |
If the air flow is constant and the liquid is constantly heated it starts to boil, when the condensation front reach the ignition source it starts to
burn without explosion, at this state no matter how much air I pumped through the boiling liquid I could not make an explosion.
|
This sounds like holding a tiger by the tail. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2818
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Two methods not tested:
* Oxidative decarboxylation of lactic acid by bromine water: there's an entire PhD thesis discussing the mechanism of this particular reaction (alpha-hydroxyacids and aqueous Br2) which is probably helpful.
Bromoacetaldehyde, a lachrymator, may be produced in some amounts as a byproduct, which is the major downside of this route. However in general
acetaldehyde should boil away as quickly as it is formed if the reaction is somewhat warm, and bromine isn't fun to breathe either.
* Triketone Strecker degradation of alanine by dehydroascorbic acid or alloxan; the former is produced by oxidizing ascorbic acid (various
possibilities: H2O2; MnO2; etc), the latter by oxidizing uric acid with nitric acid. Alloxan ingestion causes permanent diabetes, so don't.
Dehydroascorbate is unstable and must be produced in situ; it is stabilized by acidic conditions.
* Hypochlorite Strecker degradation of alanine; similar to the above but may produce chloroacetaldehyde as a byproduct.
[Edited on 26-3-2015 by clearly_not_atara]
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Magpie, the tiger did not bite! I did a run today with methanol (Cu catalyst), I let air in below and above the filament also, honestly I did not
notice the difference, there was a strong smell of HCHO in both cases.
c n atara I read somewhere that alloxan forms in food that is bleached, like flour and some sugars also.. so like it or not we eat it anyways
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
You have developed quite a technology there - well done. I will continue to watch from the door. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
..
12
13
14
15
16
..
20 |