| Pages:
1
..
10
11
12
13
14
..
77 |
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I have a nice little lamp, but if I want to run a reaction with semi-deep UV it would either need to be emersed in solution and protected with quartz
or outside of a solution which is inside of something quartz. The quartz part is where things get tricky and expensive.
Also very nice terbium salt there B&F  . .
[Edited on 15-5-2014 by smaerd]
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
Heres a little quiz: What camera did I use for these macro shots?
   
They are KMnO4 crystals
[Edited on 15-5-2014 by Pyro]
all above information is intellectual property of Pyro.  |
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I looked at the match:
Just a few properties.
Nokia Lumia 620
1/30 s exposure time
Aperture F2.4
ISO 100
Colorspace sRGB
I can give more properties, but these are the most important ones.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|


Advanced chemistry: Growing crystals of a trifluoromethylthio substituted aromatic hydrocarbon.
Compounds that contain a trifluoromethylthio group (–SCF3) are found in many pharmaceutical and agrochemical products. Owing to their
highlipophilicity and hydrophobicity, aryl trifluoromethylthio-ethers (ArSCF3) have attracted increasing attention from synthetic chemists to develop
a efficient, high-yielded, and economical preparation of these molecules.
These crystals were prepared from an aryl thiolate with trifluoromethyl iodomethane (ICF3) what is a highly poisonous, carcinogen gas.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
Woelen, that was a lot faster than I expected!
I was amazed that these results were possible on a cell phone camera.
PS:where did you find this info?
all above information is intellectual property of Pyro.  |
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|

Electrolytic Copper Sulphate (creating) cell (per NurdRage) been running all afternoon in one of girly girls bud vases....started looking pretty so
pulled the electrodes and took a back lit pic.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Arkoma, looks nice! Is the vase yours?  Any flowers may not take copper sulfate
(which happens to be sold as root kill) too well! Any flowers may not take copper sulfate
(which happens to be sold as root kill) too well!
Quote: Originally posted by Pyro  | Woelen, that was a lot faster than I expected!
I was amazed that these results were possible on a cell phone camera.
PS:where did you find this info?
|
He likely found it in the picture itself. Some picture formats store information on how a photo was taken, if flash was used, etc. It's all in the
file. Was that how you found it Woelen?
By the way, when you all buy chemicals, have you gotten any with nice crystals already? I got some ferric Ammonium Sulfate with some nice crystal
lumps (single, imperfect crystals) over 1.5 cm3.
[Edited on 5-17-2014 by The Volatile Chemist]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
LOL, no. It is my daughter-in-laws. The way it layered out and how I could see the anode actually pulling the blue tail of copper
ions towards it was "purty". The good camera is out of town ATM--took that with daggum laptop.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Match head burning @ 4,000 frames/second
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Wow, that's great! Thanks for posting it. I'd seen something like this, but much faster.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Wow, that was pretty cool. Here's some other cool slow motion videos by the same channel:
Lightning storm
Fire cracker
Very cool unknown fire balls
[EDIT] Can anyone guess what that last one is?
[Edited on 19-5-2014 by Zyklonb]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I'm the 20000th viewer of this thread!
Two telescope shots. The first one is a crow I photographed with my telescope.
<img src="http://i.imgur.com/ELsu3jG.jpg" title="I also have a photo of a dove." width=800>
The second one is Jupiter, seen during the daytime. I couldn't see its moons.
<img src="http://i.imgur.com/w7p6O15.jpg" title="The best time to do this is when the moon passes directly over or under Jupiter." width=800>
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice pictures!
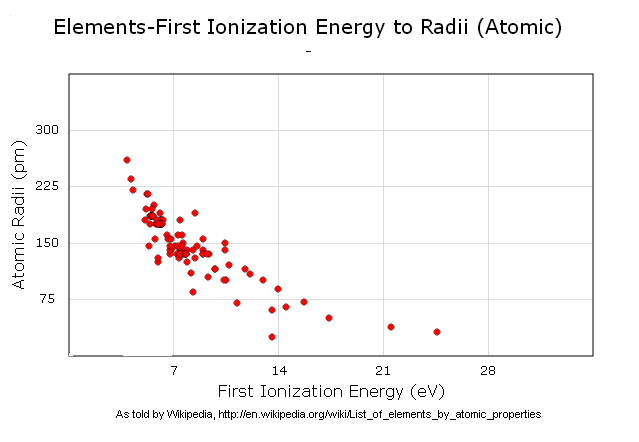
An interesting graph I made (Using a Firefox Plugin)
[Edited on 5-21-2014 by The Volatile Chemist]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
@ Brain & Force. Nice. Have done some digizooms myself. 8" Celestron with a toilet paper tube spacer and duct-tape holding my little kodak
easyshare. (click thumbs for bigger image)

Jupiter and three of the Galilean moons

Our moon

Photomicrograph of a piece of complex sulfide ore, using the same jury rig

Couldn't catch the true beauty of this Chrome/Nickel Chloride solution I got out of my salt bridge electrolytic cell this morning. Creme de Menthe,
anyone?
   
(flower pot cell with cheap stainless steel anode, carbon rod cathode and NaCl electrolyte in both halves.)
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I got one too! It's a black tube; don't know when it was made (I'm guessing the 90s) and it's on a fork mount.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|


Before and after the sublimation of ferrocene.
When we heat ferrocene (an organometallic compound with the formula Fe(C5H5)2, a sandwich compound) in vacuum, it sublimes and could be condensed with
on a cooled glass surface to form perfectly pure needle like crystals (as seen on the bottom picture).
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
A sublimation that works nicely- a rare occurrence.
Way back when, I supervised an undergraduate lab that made ferrocene. Despite their sublimation, it generally looked like the stuff in the bottom of
your second picture. I would tell them that if they wanted to see pure ferrocene, they should closely observe the crystals that formed halfway up
their capillary tube after they measured their melting points.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Wish I could say its mine, but its not. Belongs to one of my best mates.

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
But anyway, you have access to it and you can use it as well, no? 
Ethyl acetate layer, the yield of reaction was about 56%..
<img src="http://chem.pieceofscience.com/wp-content/uploads/2014/05/layeeeers.jpg" width="600" height="900">
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice, Hegi! What process did you use?
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
I refluxed mix of 30 ml 98% acetic acid with 30 ml of 95% ethanol and about 8 ml of concentrated sulphuric acid for about 45 minutes, then washed it
with sodium carbonate solution to get rid of free acetic/sulphuric acid. Separated layers. Into the organic layer I added calcium chloride to minimize
the amount of water and redistilled the product.
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
A 2 gram piece of terbium from the 6 gram piece I had, viewed under a dissection microscope. The dendritic structure of distilled terbium is easily
apparent. The yellowness isn't the terbium, it's the color of the light on the microscope.
<img src="http://i.imgur.com/VqWZULj.jpg" title="SOMEBODY GIVE ME MORE TERBIUM! please? Pretty please?" width=800>
And the setting moon.
<a href=https://www.youtube.com/watch?v=X0nEkkpShsg&feature=kp><img src="http://i.imgur.com/IkDMOS3.jpg" title="I need one of those
devices that hooks my camera securely onto my eyepieces." width=800></a>
[Edited on 4.6.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice pictures! Once I move my microscope pictures over to my desktop, I'll post a few of some microscopic crystals. Sad, my mic. camera has better
res. than my digital camera...
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
http://iconosquare.com/viewer.php#/detail/730604564436500586...
The oxidation states of copper. It's amazing how different they look!
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

Things what never-ever gets boring: fun with fluorescent dyes!
The red color is emitted by a fairly common dye: Rhodamine B. The green color is a bit more interesting, it is emitted by merbromin, an organomercury
chemical:

Merbromin is one of the best antiseptics and it is still used in several countries, but because of its mercury content, it is no longer sold in the
United States, Germany, or France. When used as a topical antiseptic, it stains the skin bright red and it is quite hard to remove. Luckily it is only
used as a 2% solution, or more dilute, and since it is not readily absorbed by the skin, it is perfectly safe to use.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
| Pages:
1
..
10
11
12
13
14
..
77 |