| Pages:
1
..
10
11
12
13
14
..
27 |
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Rosco Bodine  | | Interesting. Did you try conversion of the tris(hydrazine)zinc nitrate (ZHN) to a perchlorate derivative by mixing with 2 mole equivalents of ammonium
perchlorate. I am thinking that a double decomposition may result leaving ammonium nitrate in solution while precipitating tris(hydrazine)zinc
perchlorate (ZHP) but I have no solubility or other data to confirm the validity of this idea. |
No, I haven't. It is insoluble in water, and hot water decomposes it (hot water also slowly decomposes NHN). It is soluble in conc. aq. NH3 though.
But I would be weary of forming any kind of perchlorate here considering instability of the copper perchlorate complex and dangerous sensitivity
described above on the nickel perchlorate complex. I've seen no data on it.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@AndersHoveland
What is the basis for NHN - AgN3 to be characterized as a clathrate?
There are a lot of coprecipitates that are definitely not clathrates, and
that would seem to be the case here .
[Edited on 28-10-2011 by Rosco Bodine]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Try that for yourself and see if it's true. I couldn't even get it to detonate under confinement.
Note: yes it can explode when heated but these are likely in what I would call at least larger amounts. Probably gram amounts. It has likely a higher
critical mass than many other primaries.
[Edited on 28-10-2011 by Formatik]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Formatik  | Recently made tris(hydrazine)zinc nitrate (ZHN). It is a very shiny, glittering compound (every crystal glitters) with a beige tint. I made it by
mixing Zn(NO3)2 aqueous from zinc and nitric acid, with conc. aq. NH3 which was added until the Zn(OH)2 precipitate redissolved and the liquid took on
a light yellow color to form a clear solution, then added about the same volume of 55% N2H5OH and boiled it on low boil until crystals started
appearing where the boiling was stopped. Then let it crystallize, washed it several times with alcohol. The compound is also mentioned in Gmelin.
It is an insensitive initiator, it hasn't exploded in any heating tests done on small amounts, like over a flame, in flame directly, etc. It doesn't
even flash like tris(hydrazine)nickel nitrate (NHN) does. But it does burn very fiercely, very nicely actually even though it leaves behind a lot of
white ZnO. It burns splendid with an interesting bluish-white-orange flame. Sprinkling it onto aluminum foil and wrapping this up into a fuse caused
it to burn completely to the end when one end was lit. It does have some energetic properties, but needs to basically be blasted itself.
|
Please attach that Gmelin excerpt if you have it.
You describe boiling the solution on a low boil until crystals begin to appear. What I was thinking was to add a boiling hot solution of ammonium
perchlorate before the point where a low boil would make crystals begin to appear, seeking possible precipitation of a less soluble perchlorate
derivative via double decomposition of the nitrate.
The reaction mixture you describe is already a solution of ammonium nitrate and probably diamminezinc nitrate or diamminezinc hydroxide, so the
presence of ammonium perchlorate may cause the perchlorate salt to precipitate.
I know this works for tetramminecopper perchlorate, but haven't tried the hydrazine complex for any of these.
Generally though not always, the perchlorate salt will be less soluble so that will be what precipitates.
[Edited on 28-10-2011 by Rosco Bodine]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Perchlorate complex salts of hydrazine are dangerously friction sensitive. Hydroxylamine perchlorate is very sensitive, having a drop height value of
only 2cm. Hydrazine perchlorate is also much more sensitive than hydrazine nitrate, although I do not know exactly how much. Woelen has demonstrated
that it can at be heated over a flame in a metal spoon without detonation, but I would not want to risk trying this. It seems that compounds that
contain perchlorate together with either hydrazine or hydroxylamine (which are reducing agents) are dangerously friction sensitive. I would wonder
about nitroformate salts instead of perchlorate, since hydrazinium nitroformate is apparently stable enough for use as a rocket oxidizer.
Nitroformates are more powerful than nitrates, and almost as powerful as perchlorate. However, the sensitivity and stability of complex transition
metal salts (especially Cu, Ag, Hg, Pb) of nitroformate may be completely different, due to the unique chemistry of the nitroformate anion.
Whereas trinitromethane, even and especially when dissolved in an organic solvent is dangerously sensitive, its nitroformate salts, including
even those of hydrazine, are much more stable. One difficulty of synthesis may arise in the fact that trinitromethane itself will oxidize hydrazine
(or its salts). The trinitromethane must be added in the form of its nitroformate salt.
[Edited on 28-10-2011 by AndersHoveland]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@AndersHoveland
Zinc diammine Perchlorate is actually used as a phase stabilizer at 1.8% content in NH4NO3 oxidizer based solid rocket propellants, according to
US5071630
You often make generalizations or even specific statements about things which are simply inaccurate, the dubious clathrate is a recent example, and
you continue opining here about the hydrazinium complexes, making generalizations which are somewhat or altogether dubious generalizations.
You go through these threads with a broad brush pseudointellectualizing about things you really don't know, and I for one wish you would stop it with
the pretended knowledge, and qualify what you think by just saying, "I think" ...but it may not be so, as a kind of disclaimer 
Attachment: US5071630 Zinc diammine Nitrate.pdf (489kB)
This file has been downloaded 974 times
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | Zinc diammine Perchlorate is actually used as a phase stabilizer at 1.8% content in NH4NO3 oxidizer based solid rocket propellants,
|
Zinc diammine Perchlorate is an ammonium complex, not a hydrazine complex. There are no compatibility problems between perchlorate and
ammonia. Indeed, ammonium perchlorate is very stable, virtually impossible to detonate by itself.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Do you have specific information on the hydrazinium complex of zinc perchlorate?
It is possible it could be unacceptably sensitive and it is possible it may not be unacceptably sensitive. Unless there is a specific reference for
it, then why make such generalizations ? The hydrazinium zinc perchlorate is described in the following references, which may provide some reliable
information.
W. Friederich and P. Vervoorst, SS 21, 49 (1926)
SS = Z. ges. Schiess-Sprengstoffw.
CA 21, 1184, 1927
Semicarbazide perchlorate complexes could also be interesting ....but there is nothing I can find about them.
[Edited on 28-10-2011 by Rosco Bodine]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
It's attached below. The original description goes: a clear solution of Zn(NO3)2 in NH3 and N2H5OH is heated on the water bath, causing a brown
coloration and separation of a skin of black metal particles (I didn't see that). Upon cooling the compound separates as a white crystalline powder
(it's whitish). The Franzen and Mayer reference also mentions it burns with a green flame, I suppose there is some green in its flame also. It burns
brilliantly once ignited.
| Quote: | | You describe boiling the solution on a low boil until crystals begin to appear. What I was thinking was to add a boiling hot solution of ammonium
perchlorate before the point where a low boil would make crystals begin to appear, seeking possible precipitation of a less soluble perchlorate
derivative via double decomposition of the nitrate. |
Maybe it could work, but I wouldn't be willing to risk the glassware on it.
Attachment: Gmelin, Zn, 156.pdf (386kB)
This file has been downloaded 1039 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Thanks for the Gmelin page.
You could put your hot NH4ClO4 solution in a foam coffee cup and dribble in the other hot component solution .....no glass at risk.
Another possible way of doing this would be to react Calcium Hydroxide in slight excess with Ammonium Perchlorate and heat to boiling to drive off the
ammonia, filtering the residual solution of Calcium Perchlorate. React that solution of Calcium Perchlorate with Hydrazine Sulfate and filter out
the Calcium Sulfate to leave a solution of Hydrazine Perchlorate. Then you could add your metal complex candidate as an ammonia solution of the metal
nitrate.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here are a few deflagrations of the aforementioned hydrazine compounds:
Tris(hydrazine)nickel nitrate (NHN):
Deflagration to heat and flame:
Tris(hydrazine)zinc nitrate (ZHN):
Deflagration to heat and flame:
The same aforementioned fuse of ZHN is also in one of the videos above. It burned to the end. I'm not sure how reliable it is yet though. And around
30mg of the ZHN intermixed with about 30mg Ag2C2.AgNO3 into a small pile on an iron plate gave a very loud report on contact with a long wooden match
flame (originally Philou's idea to mix the nickel salt with acetylide complex).
Concerning hydrazine toxicity, this works by inhibiting vitamin B6. Hydrazine drugs also inhibit vitamin B6. Vitamin B6 ingestion can offset some
hydrazine toxicity, however too much vitamin B6 ingestion can cause permanent nerve damage so it has to be very carefully dosed. Respirators work
pretty good against vapor. But they do nothing to protect the eyes, which hydrazine damages.
Below is the aforementioned reference claiming spontaneous deflagration and detonation of the nickel complex. It sounds to me like they had some kind
of nasty contaminant, perhaps in their nickel source (copper maybe) in their lab. I had it sit around for about three weeks and it never misbehaved.
Copper would not be readily discernible in their original nickel but would impart a green color to the lilac salt (their impurity maybe more bogus
than copper). I've ignited 1.0g in the open and it only flashed, yet they had a violent spontaneous detonation with only 1.5g of the unconfined salt.
That's not the NHN I know.
Attachment: J. Chem. Educ. 32 [1955] 24.pdf (1MB)
This file has been downloaded 1084 times
[Edited on 10-11-2011 by Formatik]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Studies of Complex Perchlorates
http://www.dtic.mil/dtic/tr/fulltext/u2/607947.pdf
Studies of Complex Perchlorates (final)
http://www.dtic.mil/dtic/tr/fulltext/u2/634105.pdf
Chlorates & Perchlorates their Characteristics & Uses
http://www.dtic.mil/dtic/tr/fulltext/u2/318741.pdf
Chlorates & Perchlorates Their Manufacture Properties & Uses
http://www.dtic.mil/dtic/tr/fulltext/u2/242192.pdf
Related Post _
http://www.sciencemadness.org/talk/viewthread.php?tid=1081&a...
[Edited on 20-12-2011 by franklyn]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Some more thoughts on the previous nickel hydrazine nitrate:
The spontaneous explosion of the previous nickel hydrazine nitrate is a complete mystery. Rather than admit the possibility of having impure material
in the facility, they speculate the presence of some kind of higher complex, noting an excess of hydrazine, or that residual N2H5OH did some kind of
funky stuff. I think I may have read Franzen and Mayer explicitly doubting any higher complex possible. Mine also smelled of hydrazine for a few days,
but I doubt the presence of a higher complex. It's just excess hydrazine, hydrazine being hydrazine and lingers around for a few days.
The previous crude test of initiation using HgDTZ may not have worked well at all, because I didn't think of the action of a powder on top of another
simulating the effect of confinement, but it does. Initiation of ZHN and NHN was done with 0.3g PbN6 in the Journal of Hazardous Materials
171 (2009) 1175–1177, which showed maximum pressure generation of 86.9% and 105.0% of TNT for the zinc and nickel salts
respectively.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Random Idea
Hypofluorous Acid (HOF) CAS 14034-79-8
Formed much the same as the Chlorination of water H2O + Cl2 => HOCl + HCl
except it only forms on ice and is only barely stable at cryogenic temperature.
If mixed with Nitrogen Trifluoride may perhaps reform as
TetraFluoroAmmoniumoxyhydride (NF4OH)
The Lewis structure is perfectly satisfied manifesting a TetrafluoroAmmoniumoxy
cation and hydride anion.
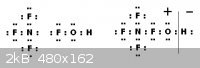
http://en.wikipedia.org/wiki/Hypofluorous_acid
http://accessscience.com/content/Hypohalous-acid/334300
http://pubs.acs.org/doi/abs/10.1021/ja00327a016
http://www.sciencedirect.com/science/article/pii/00404020778...
http://www.ncbi.nlm.nih.gov/pubmed/18355062
Properties
http://www.chemeo.com/cid/26-601-5
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Formatik, please, upload your videos on MediaFire next time if you can. It is free and I must not wait for download and all that crap. Thanks 
Nice videos, btw.
[Edited on 29-12-2011 by Adas]
Rest In Pieces!
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Thanks. Ah yes, mediafire is much better.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
double salt lead bromate acetate
Here is an obscure explosive double salt. It is doubtful that it has any practical applications, probably having poor chemical stability in storage
because of the bromate.
http://www.youtube.com/watch?v=_Xx-zhALHjM
| Quote: |
lead acetato-bromate Pb2(C2H3O2)2(BrO3)2 precipitate deflagrates with a yellowish-white puff of smoke. hammer drop height
sensitivity: 15-20cm. 175 g of KBrO3 are dissolved in 1.5 L hot water, and mixed with a solution of 175 g acetic acid (100%) and 260 g lead acetate
hydrate in 2 L water. The solution remains clear initially. It is then filtered, cooled down and seeded with a few crystals of lead bromate while
rubbing the side of the glass vessel with a glass rod. Soon after, the solution becomes turbid, and yields a heavy crystalline precipitate, which does
not increase anymore after 12 hours in the cold. The solution above the precipitate is decanted off or extracted out, and discarded (possibly add some
Na2CO3 to salvage the remaining lead in the waste solution, in the form of PbCO3). The crystalline precipitate is washed with cold water until it is
free of acetic acid and Na/K acetate/bromate, then is hot dry air is passed over until free from moisture. Yield is only 123 g
|
[Edited on 4-1-2012 by AndersHoveland]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Making that large of an amount of a primary is never a good idea. This lead salt complex also has a thread on here.
Below is the original reference of Franzen and Mayer mentioned above and some references of similar salts.
Über die Hydrazinate einiger Metallsalze. Franzen, Mayer. Z. Anorg. Chem. 60, 247 (1908).
Verbindungen von Hydrazin mit Quecksilbersalzen. K.A. Hofmann, E.C. Marburg. Ber. 30, 2019 (1897).
Zur Kenntniss der Stickstoffquecksilberverbindungen. K.A.Hofmann, E.C. Marburg. Annalen, 305, 214 (1899).
|
|
|
NatashaJurievna
Harmless

Posts: 9
Registered: 5-2-2012
Location: Carpathians
Member Is Offline
Mood: Too warlike, not friendly
|
|
Co-ordination Compounds as Sensitizers for Percussion Cap Compositions
http://www.dtic.mil/dtic/tr/fulltext/u2/a492392.pdf
Most notable, less known compounds:
Thiourea cuprous perchlorate - cuprous cyanamide complex
Behavior on ignition: Deflagrates fiercely with a dark red flame and little smoke.
Basic triethanolamine lead perchlorate
On ignition deflagrated vigorously.

|
|
|
NatashaJurievna
Harmless

Posts: 9
Registered: 5-2-2012
Location: Carpathians
Member Is Offline
Mood: Too warlike, not friendly
|
|
BTW, German patent DE922216 says about the heavily oxygen deficient basic triethanolamine lead perchlorate that "unter Einschluß detonierende Substanz" - detonating
substance under confinement.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Tetramine Zinc Permanganate
Do not know if this complex salt has been mentioned before, here is a translation from http://www.pirotek.info/Vv2/TeAZM.htm
| Quote: |
Tetramine Zinc Permanganate - [Zn (NH3) 4] (MnO4) 2 – is an ammonia complex salt, which is a relatively weak but also sensitive initiating
explosive. It has an oxygen balance of -4.3 - 8 6% (since the reaction products contain a mixture of both MnO and Mn2O3), and the volume of
detonation gases about 483 l / kg.
Tetramine Zinc Permanganate is unstable in storage, gradually decomposing with the loss of ammonia, resulting in a characteristic odor, accompanied
by self oxidation-reduction. The latter reaction is accompanied by the release of nitrogen, water and ammonia and, because this reaction is
autocatalytic, if not properly stored it can lead to an explosion, both because of the ignition, and just because of the increase of pressure in a
sealed vessel. Keep substance preferably at low temperature in a dry atmosphere, as in the presence of moisture it is less stable than ammonium
permanganate.
The solubility of Tetramine Zinc Permanganate in water is very small - the reaction of its formation is used in analytical chemistry (microcrystalline
microscopy) for the detection of zinc (with a detection limit of 1:7000 ). As a consequence, from a solution of salt precipitated in the form of fine
black powder, shiny needle crystals are formed only with careful crystallization from relatively dilute solutions. In general, due to low solubility,
synthesis of Tetramine Zinc Permanganate is simple.
Required equipment: chemical glasses (or better disposable plastic) , refridgerator (or freezer), and desirable but optional, an electric mixer and
filter.
Materials needed: potassium permanganate, zinc oxide and zinc chloride (or nitrate), ammonium chloride (or nitrate), 10 - 30% aqueous ammonia
(ammonia), distilled water.
Stages of Preparation:
1) In 100 ml of distilled water dissolve 4g KMnO4; to accelerate the dissolution it is recommended to use an electric mixer;
2) to 8 ml 20% aqueous ammonia is added 1.1 g ZnO, and 1.4 g NH4Cl (or NH4NO3); zinc oxide should dissolve completely, instead of zinc oxide and
ammonium salts can take an equimolar amount of the corresponding zinc salts;
3) The prepared solutions were mixed and rapidly cooled before freezing;
Note: The precipitation of Tetramine Zinc Permanganate takes place during mixing of solutions, cooling slows the recovery of permanganate ion and
free ammonia yields a relatively pure product.
4) The bulk of the cold solution is poured, and the residue washed precipitate on the filter, the solution to further remove the filter with the
precipitate on the stack of filter paper sheets, after which the product is loosened as soon as possible and dried at low temperature (optionally
under reduced pressure).
The result is a 4 – 4.5 g of fine black powder (yield of permanganate 85 - 95%), the presence in it a brown powder of manganese oxides indicates
that the product is partially decomposed by high temperature or duration of precipitation and drying.
In principle, the use of dilute (and not contaminated colloidal particles), the starting solution can be precipitated more pure Tetramine Zinc
Permanganate in the form of small brilliant needles, but in this case the output drops sharply.
|
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
There are some salts of salts of azotetrazole likely ammines which hold more potential I would think. Adding tetraamminecopper(II) nitrate powder to
aq. sodium azotetrazole under stirring affords a bright green solid which is uncomparably mechanically far less sensitive than the extremely sensitive
copper azotetrazole, but also less brisant.
Quote: Originally posted by NatashaJurievna  | | BTW, German patent DE922216 says about the heavily oxygen deficient basic triethanolamine lead perchlorate that "unter Einschluß detonierende Substanz" - detonating
substance under confinement. |
The copper salt appears to hold energetic properties also. It was obtained from addition of perchloric acid onto aq. triethanolamine-copper complex
and drying the liquid over H2SO4, left a deep blue solid which flashed violently with a wonderful blue light when heated over a flame. The experiment
was done several years ago and perchloric acid may have been present in excess.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Taoiseach  | DACP supposedly stands for "double azido cobalt perchlorate", the correct formula is trans-tetraamminediazido-cobalt(III) perchlorate
[(NH3)4(N3-)2Co(3+)](ClO4-). Whats interesting about this complex is the fact that it contains azide ligands, fuel and an oxidizer in a single
compound.
It is briefly investigated in a freely available paper "Lead Azide Replacement Program NDIA Fuze Conference" by Bichay and Hirlinger. It is also
discussed in more depth in several Chinese publications. I cannot download nor understand these papers but the google translation of the abstracts
gives some valuable hints. The compound is a powerful initiating explosive with properties comparable to BNCP
(bis-nitrotetrazolate-cobalt-perchlorate) and has been proposed as replacement for both BNCP and lead azide.
A German paper "light absorption of azido-cobalt-ammines" is available in reference section and sheds more light on this family of complexes.

Experimental:
2,6ml 70% perchloric acid was neutralized with basic cobalt carbonate and filtered. Another 1ml of perchloric acid was neutralized with ammonia and
the solutions combined. The resultung solution was a light magenta color .
In another beaker, 4g sodium azide was dissolved in 50ml water and 9ml 25% ammonia was added with stirring. The solutions where then combined.
Complexation occured, as evidenced by deep blue coloration of the liquid.
A stream of air was then drawn through the solution for 3 hours. During this time the color changed from a deep blue to a lavender color. After
standing for several hours, a microcrystalline powder had settled which was filtered and air dried.
|
This has to be the most interesting complex salt that has been mentioned in this thread.
There must be variation of this compound that is even better.
Perhaps hydrazine instead of ammonia? I would be hesitant to make a perchlorate complex hydrazine salt after reading about the accident with cobalt
hydrazine perchlorate, which suggests dangerous friction sensitivity.
http://www.sciencemadness.org/talk/viewthread.php?tid=14587
It is not just complex salts, because hydroxylamine perchlorate is very sensitive (drop height 2cm). But hydrazinium nitroformate
apparently is not very sensitive, as it has been researched as a solid rocket fuel oxidizer. But nitroformate probably has different compatibility
issues than perchlorate. Ammonium nitroformate appears to be the most stable nitroformate salt.
| Quote: |
Silver nitroformate slowly decomposes at room temperature, and is a very sensitive explosive. The potassium salt, KC(NO2)3 is a lemon yellow
crystalline solid that decomposes slowly at room temperatures and explodes above 95 °C. Ammonium nitroformate deflagrates or explodes above 200 °C.
Hydrazinium nitroformate is thermally stable to above 125 °C. Most salts of trinitromethane derive from the aci-form. However, the silver and
mercuric salts exist in two forms: colourless and yellow. This may indicate that two forms of these salts - nitro and aci - can exist.
|
So it would seem nitroformate would be stable in the presence of hydrazine, but not with certain metals (probably including copper).
Quote: Originally posted by Formatik  | Adding tetraamminecopper(II) nitrate powder to aq. sodium azotetrazole under stirring affords a bright green solid which is uncomparably mechanically
far less sensitive than the extremely sensitive copper azotetrazole, but also less brisant.
|
This is good to know. I would have been a little concerned about whether nitroformate would be ligand towards Co+3, as this could result in dangerous
sensitivity (nitroformate is stabilized by being an anion). But apparently complexed ammonia molecules make the transition metal ion less "noble" in
character. (difficult to explain, hopefully you understand what I mean)
Nitroformate salts are usually bright yellow in color, but might not contribute a any color if the nitroformate acts as a ligand rather than anion,
which would also presumably make it more sensitive.
Perhaps...
Hydrazine-diazido-cobalt nitroformate ?
(N2H4)2(N3)2Co[+] C(NO2)3[-]
Not sure what color it would be, but probably would have a grayish tone from being a mix of yellow and light purple. But difficult to speculate, as
complex cobalt salts can take a range of colors.
Complex cobalt nitroformate salts have already been prepared:
Journal of Structural Chemistry
Volume 32, Number 3, 339-344, DOI: 10.1007/BF00745741
Crystal structure of (4-amino-1,2,4-triazole)pentaamminecobalt(III) nitroformate [Co(NH3)5 (C2H4N4)][C(NO2)3]3
N. V. Podberezskaya, N. V. Pervukhina and V. P. Doronina
http://www.springerlink.com/content/q50434k7334m1wt6/
ethylenediamine copper(II) nitroformate
[C2H8N2]2Cu[C(NO2)3]2
Li Yang, Jin Zhang, Tonglai Zhang, Jianguo Zhang, Yan Cui
Beijing Institute of Technology, China
A quick note about the chemistry of cobalt, solutions of Cobalt(II) salts in water are not oxidized chlorine, but the presence of ammonia renders the
cobalt much more vulnerable to oxidation, even by air.
It might actually be fairly complicated to prepare a hydrazine cobalt(III) salt, since non-complexed cobalt(III) salts generally do not exist.
Oxidizing the cobalt(II) with hydrazine already in the solution would also likely lead to oxidation of the hydrazine. Perhaps Co2O3 reacted with a
solution of hydrazinium nitroformate, hydrazinium azide, and some additional hydrazoic acid would work.
Here is what the decomposition formula for the compound would be:
(N2H4)2[N3]2Co[C(NO2)3] --> CoO + 4 H2O + CO2 + 6½ N2
[Edited on 14-2-2012 by AndersHoveland]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Yes, the ammine does desensitize the original energetic compound, though it will also take away from its power unless an oxidizer or high nitrogen is
present. Another example of large sensitivity reduction is the complex of tetraamminecopper(II) nitrotetrazolate, a fairly soluble blue solid with
impact sensitivity of manyfold less than copper (II) nitrotetrazolate. Copper nitrotetrazolate is a frightening material to work with anywhere near
the dry state.
Speaking of cobalt salts, cobalt diazoaminotetrazole has very low sensitivity to mechanical action (in strong contrast to Hg, Cu, Ag, Pb salts which
are highly sensitive), cobalt azotetrazole is also of relatively low sensitivity (again in contrast to the same metals). Though both salts are not
that brisant, the latter probably more than the former. There is good potential of forming low sensitive salts with cobalt.
Thinking on the perchlorate above, how about a polyamminecobalt-azido-azotetrazolate, or nickel hydrazininium azotetrazolate.  Maybe a question too general, what is better for an energetic then, to have an
oxidizing moiety or high nitrogen content? Maybe a question too general, what is better for an energetic then, to have an
oxidizing moiety or high nitrogen content?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Yes, I was thinking about the compound with nitrotetrazolate ligands instead of azide.
(N2H4)2[N3]2Co[C(NO2)3]
(N2H4)2[N4CNO2]2Co[C(NO2)3]
But I am not sure if nitrotetrazolate can act as a suitable ligand to replace the azide. This is important because if the two anions have near the
same ability to act as a ligand, a double salt would be unlikely to form, and the ions would just precipitate out as separate crystals of two
different compounds. There is also nitrotetrazole-2-oxide that exists. So I suppose this would be possible,
(N2H4)2Co[CN5O3]3
One of the advantages of cobalt is that it would bind to the hydrazine very strongly. For example, the ammonia complexed in tetramine cobalt chloride
do not come off even if the compound is dissolved in hydrochloric acid, and indeed the complex salt can be crystallized out of a solution of
hydrochloric acid. The Co+3 ion is very acidic, one of the reasons that Co(II) salts will not be oxidized in the absence of ammonia. So no worries
about hydrazine decomposing out from the complex salt.
Hydrazine is much more energetic than ammonia, I was just thinking about how to combine several strategies together into one compound: a complex
double salt with hydrazine, azide, and nitroformate.
Quote: Originally posted by Formatik  |
cobalt diazoaminotetrazole has very low sensitivity to mechanical action (in strong contrast to Hg, Cu, Ag, Pb salts which are highly sensitive),
cobalt azotetrazole is also of relatively low sensitivity. There is good potential of forming low sensitive salts with cobalt.
|
Is the cobalt in cobalt diazoaminotetrazole in the +2 or +3 oxidation state? formula?
Also that just plain cobalt azotetrazole, or the tetramine complex with cobalt?
That would be amazing if it were just plain azotetrazole that only was not especially sensitive. Would that not mean that the ammonia complex of that
compound would be even less sensitive? Or would there be no room for the ammonia to complex?
That is a complex answer. My opinion is that it is best to combine both.
[Edited on 17-2-2012 by AndersHoveland]
|
|
|
| Pages:
1
..
10
11
12
13
14
..
27 |