| Pages:
1
..
9
10
11
12
13
..
18 |
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Geocachmaster
I have a similar hotplate,
the temperature controller needed multiple re-adjustments  so I used one of the
cheap controllers http://www.ebay.co.uk/itm/161843931556?_trksid=p2060353.m274... so I used one of the
cheap controllers http://www.ebay.co.uk/itm/161843931556?_trksid=p2060353.m274...
In use with a2l flask covered in Al foil

Top view after modification

The controller in the bottom part of an old plastic box

Modified hotplate internals

The hotplate now has better control all the way to maximum, which is higher than before..
[Edited on 7-2-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Sulaiman
That's a super good idea! I actually just bought a 2000w (I think) ac voltage control for use with my heating mantle ( ) and other projects. I think I'll put it to good use now! ) and other projects. I think I'll put it to good use now!
The heating control on that hotplate is definitely crap, either too hot or too cold.
[Edited on 2/7/2017 by Geocachmaster]
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
the controller in the photo's was ordered at the same time as a 500ml heating mantle element,
the hotplate died more recently, 
there is another controller in the post somewhere between China and me
(and a 'spare' heating element, even though construction not started yet 
P.S. for good performance a batch fractionating column needs very slowly changing temperatures, boil-up rates etc.
I have considered
. a mini-mercury-manometer to control heating based on column differential pressure
. a rex-c100 pid controller
. a £1.24 incl. p&p triac 'dimmer' ... perfect ! for fixed boil-up rates 
[Edited on 7-2-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
At least as they come, those hotplates have to bee THE worst I've ever had the displeasure of using.
Had one, and ended up tossing it just recently. If its what it looks like (I can't remember the brand, its been around a while and any lettering had
long since been eaten off by various corrosives ) )
But the temperature was thermostatic, and either on or off, cycling in pulses to attempt to regulate the temperature. A real piece of shite and with a
tendency to trip the circuit breakers unless very carefully adjusted. Got dumped in the bin the moment another became available again. And the one of
those I had was worse than unpredictable. It was the damn devil, and has been responsible for a volcanic unpleasant reaction sudden superheating which
resulted in a phosphorus volcano shooting up and out of the condenser, as well as three other times. Once the result was a huge, expensive, if rather
pretty, although pretty noxious as well, sodding great gout of subliming iodine vapor, looked as though iodine had been substituted for Cl2 in a damn
WWII trench, and the other one ended up starting diethylene glycol smoking and fuming at a roiling boil in a heating bath, which whilst rather,
well..not exactly penetrating, and didn't have that sheer carrying or persistency of say, some of the foul-smelling haloalkyl sulfur heterocyclic
monstrosities spawned by overheating 1-chloro or 1-bromothiazol-2-ethanes which were just brutal, the diethylene glycol heating bath, which happened
to contain salts, CaCl2 and NaCl, due to a former life as a coolant bath with ice and MeOH/EtOH, good god the smell. It was subtle, but odious, a
really nasty sickly sweet 'ripe' stench. Not like rot, exactly, but like fruit going rotten without the fruity ester components, and putrid. Stagnant
and sickly. Not terribly strong as such, but even faint sensation of that was enough to nearly make one vomit.
The plate eventually grew to be so despised that it went into the trash. The death knell sounded for it the time dipropionyl and dibenzoylmorphine
sulfate both almost ended up burnt to a crisp during a sudden temperature spike. Luckily most of the esters were able to be saved, but from
70-something 'C to over 250 'C in no time flat. Luckily the reaction involving the esters was being watched like a hawk due to the preciousness and
temporary irreplacability of the substrate and the vessels were snatched, hot as they were, off the place before the substrate was deep-fried in acyl
halide. Bugger the hands. The yield had to be saved in that case. Not easy to keep hold of and it had a good go at shrink-wrapping a pair of hands in
shrunken gloves. A good deal of swearing followed to say the least, although at least, given the specific activity of the compounds produced, at least
the actual pain from grabbing the bloody well hot flask necks stood little chance of standing up to 3,6-dipropionylmorphine. Little yield left
afterwards, due to its being needed to sooth the hands that rescued it, but some is better than none. dipropionyl and dibenzoylmorphine turning to
carbon..now that would be a sight to make any chemist weep. After that, it went to another hot place. This one with lots of fire and brimstone, from
whence it came and where it deserves to be.
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
These hotplates are definitely horrible for temperature control with the included thermostat. I find it useful for dehydrating stuff that can't
decompose/burn. For example if I'm out of anhydrous magnesium sulfate and don't have a lot of time I just throw a bit in a beaker and turn it on full.
Also the heating surface is covered with some kind of paint which can stick to glass; it's awful to clean off. I bought a ceramic top hotplate stirrer
when I could, but it blew half of this years chem budget.

Much better
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
They aren't bad at temperature control if you use a heating bath, but it's easy to burn stuff placed directly on the hotplate's surface. I used a
table salt heating bath with mine last week, and it worked great.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I had a lot of use out of my cheap hotplate before I modified it,
as above, I had two major problems;
the on/off/on/off.. temperature control has a large hysteresis,
so distillations would surge, then die down, then surge, then die down....
OK for a simple distillation but terrible for fractionating.
Possibly due to micro-welding of the switch contacts, the hotplate would occasionally 'stick' on too long and massively overheat for a while.
Converting to a 'dimmer' has transformed the hotplate !
I now feel confident to leave distillations running with very little monitoring,
nice steady boil-up rates.
I recommend this modification to anyone with a little electrical wiring expertise,
even if you think your hotplate is ok ... try a modified hotplate
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think I'll definitely pick up some of those dimmers... while I've been trying to cut back on brushed motors, I still have plenty of resistive
heating. Why use a $150 variac for your heating mantle....
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
My current mag stirplate that needed a repair (he's no electrician generally, not with his math) is now thankfully repaired, again with a modification
like that, converting the snap-switch (preset levels, without intermediacy, at first) to a dimmer. Still getting used to the hack of the controls. But
MUCH better.
The other one had to go though. Was going to rip the resistance wire out of it, but one night, found it had just been thrown out. Fucker was too
dangerous to plug back in and once his old man repaired the other one and did that little hack on the controllers, it went before he had chance. Not
that he misses it, and not like a bit of nichrome resistance wire is going to break the bank of ever needing to buy any.
First time he knew of that sudden massive overheating. Elemental phosphorus was one of the reagents. doing an RP-I2 reduction of the substrates he was
working on at the time, and lets just say, the cleanup wasn't much fun. PI3, I2, burning phosphorus that thankfully, didn't QUITE hit the ceiling. Not
far off though, using a long, thin necked flask at the time, a side-arm distillation flask another time was even less pretty (one with an inbuilt long
glass side-arm coming off the neck about an inch or two down from the top of the neck. If the top end had been sealed, that would have been...well
glad not to be standing too close due to the resulting sideways direction of the jet of reactants
[Edited on 8-2-2017 by tsathoggua1]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I had been doing camera work, including a bunch of chemistry videography & photography, using a clamp, stand, and (ugh) selfie stick to hold my
camera as a makeshift tripod. It was ok, but still pretty wobbly and inconvenient. I picked up a tripod not too long ago at the local Really Free
Market, and decided to retrofit the selfie stick to the tripod so that it could hold my camera phone. Here it is, after sanding down rough edges and
epoxying an appropriately-threaded nut onto the phone holder:

al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
My first attempts as making glass pipettes. The top two are made from glass droppers (right) and the bottom two are made from glass tube. The bottom
ones are blackened because I pressed the hot glass on a piece of wood to flare the edges, got a nice deposit of soot. Would metal cause cracking?
I heated the last 1.5cm of the tube while rotating until the hole closed and the glass softened enough for it to droop when rotation stopped. Then
using a pair of tweezers I grabbed the end and carefully pulled it out. The faster pulled the thinner the tip will be.


My favorite one
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ok. This is worth thinking about.
https://youtu.be/ispolAHB4jA?t=1m56s
|
|
|
Clonejeffie
Harmless

Posts: 31
Registered: 21-2-2017
Member Is Offline
Mood: Curious
|
|
Homemade magnetic stirrer 9v bat and computer fan
    
|
|
|
Clonejeffie
Harmless

Posts: 31
Registered: 21-2-2017
Member Is Offline
Mood: Curious
|
|
Homemade magnetic stirrer 9v bat and computer fan
      
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I made a flexible stir shaft by twisting a length of electrical tape into a core and wrapping several layers of electrical tape around it. It is super
ghetto, but it works.
|
|
|
haita
Harmless

Posts: 4
Registered: 13-9-2017
Member Is Offline
Mood: No Mood
|
|
DIY lab equipment
How many of you have DIY centrifuges/thermocyclers/shakers/etc.? This is a thread to post pictures of them and share instructions for building
equipment. I don't personally have any self-made tools, as I have access to a pretty good lab, but here's a link to a dremelfuge and a couple of PCR ideas.
|
|
|
gdflp
|
Threads Merged
14-9-2017 at 04:51 |
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I tried to make a ball mill from a peanut butter jar, the bearings out of a dishwasher, and a severely under powered motor. It lasted for a good 20
seconds.

Next I made an HHO generator from several plastic containers hot glued together. I used nails as the electrodes and baking soda for the electrolyte
so it did not last long either.

When I first got into element collecting I made a box so I could safely electrolyze NaOH. I did not know that NaOH was deliquescent at the time so
when I was done I just put it at the back of my workbench and left it for a few days. When I came back it was a fizzing swollen caustic mess. Good
times.

Best of all though is my homemade liebig condenser. I cut the ends off of disposable test tubes with a diamond cutoff wheel and butt jointed them
together with my blowtorch. I made a cooling jacket out of PVC and an adapter for my Erlenmeyer from a rubber stopper. It worked, but it leaked
horribly, it was difficult to position without breaking the joints between the tubes, and never got above a 10% yield with it. Some things are worth
paying for.
    
[Edited on 26-9-2017 by Plunkett]
|
|
|
alexzxz
Harmless

Posts: 5
Registered: 11-6-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Plunkett  | I tried to make a ball mill from a peanut butter jar, the bearings out of a dishwasher, and a severely under powered motor. It lasted for a good 20
seconds.
|
Yeah, ball mills seem so easy. But after fiddling with jars, rubber belts, caster wheels, and a few motors for months, it's still unreliable and cost
way more just buying one from harbor freight.
I eventually caved and just bought the single drum with the 20% coupon for about $30. Then bought a dual drum a year later since they're so useful.
Small ball mills aren't worth the effort, but very large ball mills (much more efficient, much finer grind) are absolutely worth it if you have the
space and time
[Edited on 18-12-2017 by alexzxz]
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I got some borosilicate drinking straws as a gift and figure they could be repurposed for something. And the little cleaning brushes reminiscent of
test tube brushes ...
  
[Edited on 5-1-2018 by Morgan]

|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
I finally tried out what I thought might be a really good airator which is simply part of a borosillcate glass tube a friend was throwing away, he has
tons of these as waste from normal working with glass tube. It has a 1/4" OD glass tube barb at the top and fits perfectly inside any 1/4" tubing -
these can be made to any side basically (smaller than the tube that is).
The tube is filled with some cotton swabs rolled up and pushed into the bottom.
I tried it against an aquarium air stone and the bubbles were about 1/40th the size of the air stone (more like a mist of bubbles, instead of actual
bubbles, the water gets cloudy with air towards the top of the water!) and gives a ton more surface area for the gas to absorb into the water. I
thought this would be good for trying to absorb NH3 into water. Will the bubbles being so fine be an issue?
If anyone is interested I could probably get a bunch of these (if anyone is interested) and even some larger ones from 3/4" to 3" ID and thick walled.
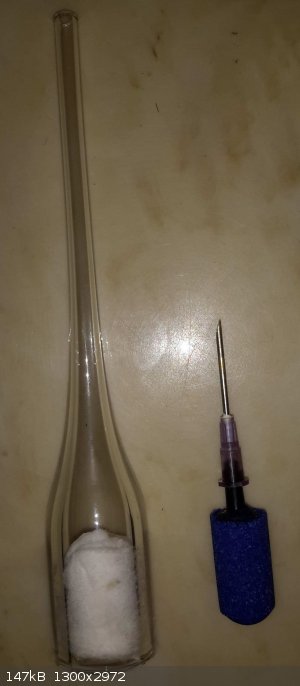
[Edited on 1-22-2018 by RogueRose]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by Geocachmaster  | | My first attempts as making glass pipettes. The top two are made from glass droppers (right) and the bottom two are made from glass tube. The bottom
ones are blackened because I pressed the hot glass on a piece of wood to flare the edges, got a nice deposit of soot. Would metal cause cracking?
|
They’re nice, I do a bit of glass work myself, usually making odd bits out of tubes. I find the best way to get the soot off is to heat it in a
strong oxidising flame - once it starts glowing red then it’ll sublime off and leave perfectly clean glass. Metal won’t cause it to crack if
it’s still pliable, but make sure that the metal is insulated or long length of metal since it can burn fingers in a matter of seconds - I have more
scarred burns from hot metal/glass on my body than I do from chemistry, which says something considering I rarely wear PPE. If I need flares, I use an
old metal thumb drive in the shape of a bullet and just press it into the opening.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Making a gas powered Bunsen burner into a portable unit:
https://www.google.com/url?sa=t&source=web&cd=3&...
It was quite easy to do and the parts are cheap too.
|
|
|
PrussianBlue
Harmless

Posts: 38
Registered: 20-1-2018
Member Is Offline
Mood: No Mood
|
|
Homemade Distillation Setup
It's nothing too fancy, but I figured I would share my homemade (janky) distillation setup from awhile back. It's certainly not an aesthetic piece,
but it successfully distilled over 50mL of concentrated nitric acid on its first use. It's surprising what one can do with a wine bottle, glass
tubing, and a propane torch.
 
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I was looking at some various small diameter tubing on eBay and it's kind of expensive for just a simple aluminum tube but found some thrift store
knitting needles in various sizes for very little - maybe of use for some projects.

[Edited on 26-1-2018 by Morgan]
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Al tubing can be had from fridges. Yards of it.
|
|
|
| Pages:
1
..
9
10
11
12
13
..
18 |