| Pages:
1
..
9
10
11
12
13
..
18 |
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Today I made a very tiny amount of silver azide, mostly to test my newly made hydrazine sulphate. I made a 50 ml solution of silver nitrite at 60 C
and mixed it with 30 ml of a dilute hydrazine sulphate solution. After a minute, a flocculent precipitate formed. I filtered and too a very tiny
sample of the wet azide on a steel sheet. Maybe a mg. Then I lets it dry, and heated with a match. I eard a very loud pop sound. I was amazed by the
sound made from a such tiny amount of material. I then poured hot sodium nitrite solution on the azide to decompose it, washed all my glass and stored
them.
Hopefully this is of interest to someone.
I never asked for this.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Nice! Its is quite powerfull, huh? EM makes me nervous. I biult a steel impact test mechanism, and am always startled by how loud just a few mg of
sensitive HE is. really puts it in perspective...
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
hydrazidocarbamoyl azide / hydrazodicarbonazide / Hydrazinodicarbonic Acid Diazide
Here's an obscure "carbonic" azide that would seem to qualify as a "green initiator" known by three slightly different names. Searches for this
compound turn up very little information.
hydrazidocarbamoyl azide / hydrazodicarbonazide / Hydrazinodicarbonic Acid Diazide
Also described on page 2 of the pdf Carbamoyl Azides (attached)
Attachment: Hydrazinodicarbonic Acid Diazide page 208 Vol 7 H-L PATR.pdf (74kB)
This file has been downloaded 951 times
Attachment: Carbamoyl Azides.pdf (709kB)
This file has been downloaded 1156 times
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by Bot0nist  | | Nice! Its is quite powerfull, huh? EM makes me nervous. I biult a steel impact test mechanism, and am always startled by how loud just a few mg of
sensitive HE is. really puts it in perspective... |
Well, it was only to test the hydrazine sulphate, but yea, very powerful. I'm not interested in EM tough. I don't really understand the interest
ether.
I never asked for this.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
more regarding Biurea / hydrazodicarbonamide
Other references are found regarding the use of Biurea as a precursor for azides, and oxidation by hypochlorite is one method. Nitrosation by HNO2 is
another. Carbamoyl azide is an intermediate product which is further hydrolyzed to hydrazoic acid. This is interesting because it occurs the
hypochlorite oxidation occurs in a reaction system that is alkaline, which increases the solubility of the biurea, and which favors hydrolysis of the
carbamoyl azide, and which favors neutralization of the hydrazoic acid as either its sodium or ammonium salt. Urea is a byproduct of the hydrolysis
of the carbamoyl azide. The nitrosation of the biurea in aqueous NaOH may also be possible using isopropyl nitrite, although no references for this
have been found.
(from earlier discussion, related)
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=6729&a...

Attachment: Darapsky Journal fur praktische Chemie.pdf (1.6MB)
This file has been downloaded 958 times
[Edited on 23-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by plante1999  | Today I made a very tiny amount of silver azide, mostly to test my newly made hydrazine sulphate. I made a 50 ml solution of silver nitrite at 60 C
and mixed it with 30 ml of a dilute hydrazine sulphate solution. After a minute, a flocculent precipitate formed. I filtered and too a very tiny
sample of the wet azide on a steel sheet. Maybe a mg. Then I lets it dry, and heated with a match. I eard a very loud pop sound. I was amazed by the
sound made from a such tiny amount of material. I then poured hot sodium nitrite solution on the azide to decompose it, washed all my glass and stored
them.
Hopefully this is of interest to someone. |
For historical reference, the experiment you describe was reported by an Italian chemist Angeli 120 years ago.
Attachment: Angeli Silver Azide Ber., 26, 1893.pdf (307kB)
This file has been downloaded 916 times
http://www.youtube.com/watch?v=KShGGElRsxU
<iframe sandbox width="640" height="480" src="http://www.youtube.com/embed/KShGGElRsxU" frameborder="0" allowfullscreen></iframe>
[Edited on 24-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Mentioned earlier in the thread is a book
Organic Azides Syntheses and Applications ( Wiley 2010 )
The 11 Mb file is too large for a single attachment so I have split the file into 7 numbered parts as attachments for this post and a subsequent post
which you may merge to recompile the book.
Here are the first 4 parts
Attachment: Part 1 Organic Azides Syntheses and Applications Wiley 2010.pdf (1.8MB)
This file has been downloaded 5247 times
Attachment: Part 2 Organic Azides Syntheses and Applications Wiley 2010.pdf (1.7MB)
This file has been downloaded 11983 times
Attachment: Part 3 Organic Azides Syntheses and Applications Wiley 2010.pdf (1.8MB)
This file has been downloaded 10461 times
Attachment: Part 4 Organic Azides Syntheses and Applications Wiley 2010.pdf (1.9MB)
This file has been downloaded 10099 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Mentioned earlier in the thread is a book
Organic Azides Syntheses and Applications ( Wiley 2010 )
The 11 Mb file is too large for a single attachment so I have split the file into 7 numbered parts as attachments for this post and the preceding post
which you may merge to recompile the book.
Here are the final 3 parts
Attachment: Part 5 Organic Azides Syntheses and Applications Wiley 2010.pdf (1.9MB)
This file has been downloaded 1393 times
Attachment: Part 6 Organic Azides Syntheses and Applications Wiley 2010.pdf (1.8MB)
This file has been downloaded 1798 times
Attachment: Part 7 Organic Azides Syntheses and Applications Wiley 2010.pdf (824kB)
This file has been downloaded 6814 times
http://www.youtube.com/watch?v=TJKu0yShERQ
<iframe sandbox width="640" height="480" src="http://www.youtube.com/embed/TJKu0yShERQ" frameborder="0" allowfullscreen></iframe>
[Edited on 24-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Inorganic Azides
Inorganic Azides Solubility Chart
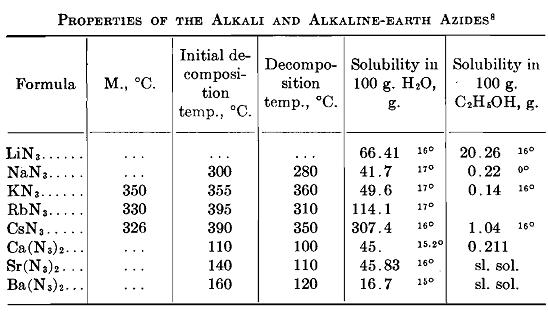
Hydrazoic Acid and Its Inorganic Derivatives.
L. F. Audrieth
Chem. Rev., 1934, 15 (2), pp 169–224
(attached)
Synthesis 26 and 27 from Inorganic Syntheses Vol. 1 (attached)
Synthesis 40 from Inorganic Syntheses Vol. 2 (attached)
Energetic Materials Physics and Chemistry of the Inorganic Azides, Fair (attached)
Attachment: Hydrazoic Acid and Its Inorganic Derivatives Audrieth Chem. Rev. 1934.pdf (812kB)
This file has been downloaded 1706 times
Attachment: Synthesis 26 and 27 from Inorganic Synthesis - Volume 1.pdf (538kB)
This file has been downloaded 1358 times
Attachment: Synthesis 40 from Inorganic Synthesis - Volume 2.pdf (472kB)
This file has been downloaded 1509 times
Attachment: Energetic Materials Physics and Chemistry of the Inorganic Azides, Fair.pdf (1.2MB)
This file has been downloaded 2001 times
[Edited on 25-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Staudinger Azide Patent DE273667
Here is an interesting idea related to the patent DE273667 which describes use of a nitrosamine as the reagent for diazotization / nitrosation of
hydrazine to form an azide.
http://www.sciencemadness.org/talk/viewthread.php?tid=3453&a...
Thanks to Boffis for translation of the Staudinger Azide patent. I changed the formatting slightly to fit it on a single page and made a pdf for it
too. (attached)
Urbanski 3 has a writeup on R-salt that provides useful information about the hydrolytic properties (attached)
Here also is an R-salt synthesis which I am not certain but I don't recall being posted before. From Journal of the Chemical Society 1889.

This idea of possible usefulness of R-salt as a nitrosation reagent is not a new idea for me. On the preceding page where I was mentioning again the
Hodgkinson patent
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
towards the bottom of that post I mentioned a discussion a decade ago at the old newsgroup alt.engr.explosives, and linked a part of that discussion
which was much more extensive. Near the very end of that thread I brought up the Staudinger patent which I referenced by the patent number and the use
of a nitrosamine, and speculated that R-salt may also work. So this is a review here a decade later with additional references and translations for
benefit. Here I will link that old newsgroup conversation, which was mostly between PHILOU Zrealone and myself. The relevant post is the third post
up from the bottom of the page
http://alt.engr.explosives.narkive.com/sJmT2yCp/interesting-...
An excerpt pdf showing the relevant post is attached.
So this discussion and review of azides has come full circle over 10 years. There's the deja vu. There is no "re" in research without a little deja
vu.
Attachment: DE273667 Staudinger Azide from nitrosamine and hydrazine patent.pdf (104kB)
This file has been downloaded 843 times
Attachment: R-salt preparation JCS.pdf (164kB)
This file has been downloaded 876 times
Attachment: German Patent 273667 A method for producing hydrazoic acid or azide salts.pdf (45kB)
This file has been downloaded 1013 times
Attachment: Nitrosamine pages from Urbanski 3.pdf (273kB)
This file has been downloaded 4187 times
Attachment: excerpt from Interesting Azide Patents ( circa 1919 ) - page 5.pdf (18kB)
This file has been downloaded 1027 times
http://www.youtube.com/watch?v=LjRuVehaXJ4
<iframe sandbox width="640" height="480" src="http://www.youtube.com/embed/LjRuVehaXJ4" frameborder="0" allowfullscreen></iframe>
[Edited on 27-4-2013 by Rosco Bodine]
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Dang That is a nice line up of useful material. Thanks for that Rosco
*One question in regards to the disposal of waste liquor : From reading Urbanski 3, I found that it is the practice to use a sol. of 15%Hno3 and
8%Nano2 to destroy the remaining azides . What is a good way of knowing if you effectively neutralized the waste ? Assuming the mother liquor was
400ml in total (the final azide produced was 5g). The wash water and dextrinated wash would be included in this to add another 400ml approx . totaling
800ml of waste water.
*Another thought concerning Agn3. ; My understandings are that it is NOT practice to recrystallize azides to purify them( due to the dangerous
formation of large crystals), mostly LA. Is this danger the same for Agn3? Urbanski stated that Agn3 is soluble in ammonia. If Agn3 was to be brought
into sol. of ammonia and recrystallized, would it become a double salt of both ammonium/silver azide? OR, would the Agn3 recrystallize into a larger
crystal formation ( could this be avoided by a cool re-crystallization of the product.)?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yeah I was hoping to find and share something useful, but am not sure if any of these references may lead to anything new. I suspect a lot of
experimentation is probably already done which didn't produce any impressive yields and those experiments were never published or are buried in
obscurity. There is always a chance of finding something new or getting new ideas by reviewing the old references.
Sodium azide is biodegradable and has been used as a soil fumigant for weed and nematode control as a substitute for methyl bromide. AgN3 is
reportedly more sensitive than silver fulminate, and has only found use in some specialized micronized particulate "colloidal" form, and even then is
still sensitive. Many references say AgN3 is treacherous, which reminds me of the similar language for copper and cadmium azides. The lead salt and
its composites still rule in terms of practicality and economy and performance.
The possibility of using nitrosamines to perform the diazotization, and the use of alternative "donors" for the hydrazine function just seemed to open
up possibilities for an aqueous reaction system method to be devised, and this idea has always been intriguing as a potential simplification. Not
only could R-salt be a potential candidate but DNPT might also work. Anyway it is good to find some ideas for experiments which have some promise and
yet are not reported.
Using methanol or isopropanol or ethanol as a solvent extraction for the hydrazine hydrate, basified with either NaOH or KOH or an alcoholate, and
then treating with an organic nitrite ester is a proven approach. But it is likely that an aqueous reaction system scheme with reasonable efficiency
can be found, and that is mainly what I have been interested in finding or devising. It is a tough nut to crack.
[Edited on 30-4-2013 by Rosco Bodine]
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Tough nut to crack is right. 
They are a intriguing field to study, and provide unique initiating property's .
You comment about the sensitivity of Agn3, was it related to impact, friction, of photo-sensitivities? It was my understanding it is almost par with
LA in power and less sensitive to the hammer drop. Urbanski #3 /pg #184 last paragraphs : state the Agn3 is less sensitive to the drop v.s LA. Also
the hammer drop needed to initiate is 5x more than Merc-Fulm.
My assumption is the photo sensitivities you speak of in terms of dangers . I dont recall however finding the friction ratings of the material in
comparison. Do you have some references to this aside from urbanski?
Also, Is the sol. of hno3, and Nano2 only needed in small amounts to kill the azide left in the waste wash? I was assuming that a 100ml sol. of 15%
hno3, 8%nano2 would be more than effective to make the waste liquor(800ml) safe to dispose of . Is this a fair assumption ?
Also thanks for the in-site on the Nan3 being a fumigant for soils , I wasnt aware of that . good to know , though.
[Edited on 30-4-2013 by pjig]
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
I guess it is the sensitivities to friction that make it more of a danger to work with.
PATR2700 Vol 1 /pg# A598
Explosion Temperature, 0 C. 297° in 5 sec
for a 0.02g sample (Ref 15) to 308° in 1 sec
for a 0.02 g sample (Ref 58)
Friction Sensitivity, extremely sens, but
more stable to friction than either Cu, Ni or
Co azides (Ref 15) (also see Ref 28)
Impact Sensitivity, 3 in with 2 kg wt and 6
cm with 1 kg wt or 41 cm with 500 g Wt vs
43 cm for LA both in BM App (Refs 28&,58)
Initiating Efficiency, see table under Mercutous
Azide (or Ref 16)
Lead Block Expansion, 22.6 cc for 2g sample
vs 25.6 cc for MF(Ref 9, p 247)
Stability, color remains white when kept in
the dark but on exposure to sunlight crystals
darken. It is stable at 75° (Refs 28& 46)
Temperature Developed on Explosion 3545°
vs 3420° for LA(Ref 16)
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Azide's decomposition is exothermic right ?
Since like, example 2NaN3 > 2Na + 3N2 , the tripple N2 bonds release more energy than the doubble ones in NaN3, so is it exothermic ? or the ionic
energy breaking need a lot of energy ?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
And do u have information for Lithium Azide on ur book ?
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Patr pg. a588
Lithium Azide (formerly called Lithium
Azoimide or Lithium Trinitride), LiN3, mw
48.96, N85. 83%; anisotropic, CO1 crysts, mp expl
115° to 29@ (Ref 1); sol in w (36. 1% at
10° and 66.4% at 160), sol in alc (20.3% at
16°) and insol in eth (Ref 1); Q? -2.58 kc~/
mol at 298°K, lattice energy 194 kcal/mol
at 298”K (Ref 21). Prepd in 1898 by Curtius
& Rissom (Ref 1) by the action of a soln of
lithium sulfate on barium azide and evapn of
the clear liq.
Explosive Properties. Li azide, although
detond with difficulty, propagates at a
velocity of 990 m/see (Ref 15). Wohler &
Martin (Ref 5) obtd an expln temp of 245°
for 0.02 g of the subst which detond violently
after 5 sec, but this compd could not be
detond by impact.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Lithium azide has only moderate explosive properties. Decomposition of lithium azide only forms lithium nitride and nitrogen. It can survive hammer
blows without detonation
Sodium azide is not itself an explosive. The main reason for this is rather simple- the decomposition proceeds according to the following equation:
2 NaN3 --> 2 Na + 3 N2
The reduction of sodium ions to elemental sodium is not particularly favorable, and this is one of the main reasons sodium azide is not explosive.
Sodium nitride does not form because Na3N is not very stable (sodium nitride decomposes into its elements at only 87 °C ).
Calcium azide begins to thermally decompose above 110 °C, and explodes at 158°, it is more explosive than either strontium or barium azide.
Barium azide is a sensitive explosive, with a drop height value of 10cm. It appears to be relatively insensitive to impact but highly sensitive to
friction. H. Ficheroulle, Mem. des Poudres. 33, 7 (1956)
The temperature at which barium azide explodes is apparently highly variable, values have been reported between 152° to 221°C.
The enthalpy of formation for barium azide from its elements is actually slightly negative, -5.3 kcal/mole. The formation of barium nitride is very
favorable, the compound having an enthalpy of formation of -89.9 kcal/mole. "Nitrogen Burning of Metals", G. Petrov, Combustion, Explosion, and
Shock Waves, Volume 11, Number 3, 309-312
The decomposition products from the explosion of barium azide leave behind a significant quantity of metallic barium, whereas the explosion of
calcium azide leaves only calcium nitride.
[Edited on 23-6-2013 by AndersHoveland]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Wow thanks.
What about Magnesium azide ? does aluminum or titanium azides exist ? if so may i know little about it  I think elemental is good. I think elemental is good.
If lithium azide was elemental decomposition, it would have been great, lithium alone could have burned by oxygen to form the second most exothermic
reaction in all of chemistry 
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
can someone reply ^
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
http://magnesium.atomistry.com/magnesium_azide.html
http://pubs.acs.org/doi/abs/10.1021/ic9614834
I found these two references with a minimal search. Dig a little harder.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Technology of the Inorganic Azides, Energetic Materials Vol. 2, Fair
http://www.google.com/url?sa=t&rct=j&q=&esrc=s&a...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Primary Explosives
http://www.mediafire.com/download/9fzstasacfqrato/Primary+Ex...
You may also like this
High Energy Materials, Agrawal, Wiley
http://f3.tiera.ru/2/Ch_Chemistry/Agrawal%20J.P.%20High%20En...
Here is an interesting Russian site
http://www.exploders.us/sprawka/26.html
Google translation link
http://translate.google.com/translate?hl=en&sl=ru&u=...
And a Google translation of the lead azide section is attached. The validity of the translation and the validity of the described processes is not
verified.
Attachment: translation lead azide.pdf (17kB)
This file has been downloaded 1990 times
[Edited on 5-9-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
memo for Dr. Klapoetke and his team
Quote: Originally posted by Rosco Bodine  | Here's an obscure "carbonic" azide that would seem to qualify as a "green initiator" known by three slightly different names. Searches for this
compound turn up very little information.
hydrazidocarbamoyl azide / hydrazodicarbonazide / Hydrazinodicarbonic Acid Diazide
Also described on page 2 of the pdf Carbamoyl Azides (attached)
|
In addition to the above compound with references linked in the post earlier in this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
There is mentioned earlier in the literature a diazonium salt of carbamoyl azide obtained by nitrosation of carbamoyl azide. It is compound IIIc on
page 385 of the following article attached below.
Über die Ultraviolett-Absorption und Konstitution von Tetrazenen aus Aminoguanidinsalzen
Ruth Hofsommer, Max Pestemer
Zeitschrift für Elektrochemie und angewandte physikalische Chemie
Volume 53, Issue 6, pages 383–387, Dezember 1949
This diazonium salt of carbamoyl azide IIIc is a monobasic cation which could also form salts different from the identified chloride, and salts such
as the picrate or styphnate or nitrate or perchlorate may be possible and may be interesting, as well as salts with various tetrazoles.
Additional context is provided by the following article attached.
Ueber das sogenannte Diazoguanidin
A. Hantzsch, A. Vagt
Justus Liebigs Annalen der Chemie
Volume 314, Issue 3, pages 339–369, 1901
On page 368 next to the last page in the lower chart on the bottom left is a "missing compound" as would correspond with the chart in the section
above, and it would seem that this missing element would be occupied by the compound IIIc, as a variant alternative outcome.
What is the stability of the diazonium salt of carbamoyl azide and if it is sufficient to provide any practical usefulness for any derivatives is
unknown. Also unknown is whether further investigation of this potential energetic compound has already been done.
Attachment: Carbamyl Azide 70 per cent yield from Semicarbazide Hofsommer and Pestemer article.pdf (518kB)
This file has been downloaded 833 times
Attachment: A. Hantzsch, A. Vagt, Annalen 314, 3, 339-369.pdf (1.1MB)
This file has been downloaded 871 times
https://www.youtube.com/watch?v=HSgVcNEUEPc
<iframe sandbox width="640" height="480" src="//www.youtube.com/embed/HSgVcNEUEPc?rel=0" frameborder="0" allowfullscreen></iframe>
https://www.youtube.com/watch?v=opleOxh0W_0
<iframe sandbox width="640" height="480" src="//www.youtube.com/embed/opleOxh0W_0?rel=0" frameborder="0" allowfullscreen></iframe>
[Edited on 23-9-2013 by Rosco Bodine]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Could I please get some information on bismuth triazide I could only find a paragraph about it. For example I know that it forms complexs however I
haven't found out what it will Form a complex with.
I wonder if anyone has ever been brave (or stupid) enough to make polonium azide.
[Edited on 17-10-2013 by bismuthate]
|
|
|
| Pages:
1
..
9
10
11
12
13
..
18 |