| Pages:
1
..
9
10
11
12
13
..
30 |
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
What is the structure of copper (II) formate? Just as the oxalate (Cu2(HCOO)4)? Or is is Cu(HCOO)2?
Or a completely different structure?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The structure of the dihydrate has been determined:
Acta Cryst. 1965, 19, 357-362. DOI:10.1107/S0365110X65003456
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
If I only want a vacuum pump for filtration (and maybe distillation) is it better to use an aspirator with a jet pump, the faucet, use a hand pump, or
buy a 100$ vacuum pump from Harbor Freight? I am concerned that the local water supply does not have enough pressure to pull an acceptable vacuum on
an aspirator.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
What is the simplest Styrene polymerization inhibitor that I can get over the counter?
Its oxygen that catalyses the reaction right? Would a small about of Ethanol or something of that sorts inhibit the polymerization? How about
dissolving it in something like Xylene so that the concentration isn't very high?
I just want to attempt pyrolysis of PS and I want to be able to store it in order to perform various experiments with it.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I am not sure but isn't hydroquinone used for this purpose?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Does anyone know where to buy 1,3-dichloro cyclobutane? Any other 1,3- derivitives would also be suitable.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by barley81  | | If I only want a vacuum pump for filtration (and maybe distillation) is it better to use an aspirator with a jet pump, the faucet, use a hand pump, or
buy a 100$ vacuum pump from Harbor Freight? I am concerned that the local water supply does not have enough pressure to pull an acceptable vacuum on
an aspirator. |
For 8 years and a lot of chemistry under the bridge I have only used a hydroaspirator. This has a lot of advantages if you have adequate water
pressure. I don't know the minimum acceptable pressure, this would be equipment and task dependent, but I'm assuming 30 psig would do. Mine will
draw down to a 50-60mmHg pressure, adequate for all filtrations and all the vacuum distillations to date. If the vacuum distillation procedure
specifies 15-20mmHg pressure I have found that my 50-60mmHg will suffice. The product just comes off a little higher in temperature. So I lose a
little more product due to decomposition.
So, unless you need a hard vacuum, or your aspirator is going to run for hours and the water consumption would be excessive, why buy a pump? If the
pump is oil lubed you'll need a trap or have to change oil frequently.
I wouldn't buy a hand pump when an aspirator is just as cheap, unless water usage is a problem.
So you see, there's no direct answer. The choice very much depends on the individual's circumstances.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I use my fridge pump, before that I used a medical diaphragm vacuum pump. I think it is cheaper to go one of these routes than setting up an
aspirator/recirculating system. On the other hand, if you just run it from your faucet, and don't care about the water usage, by all means, go for
the aspirator.
The good thing about the aspirator is you don't have to worry about sucking evaporated solvent into the pump oil, but this is mostly preventable w/o a
trap by not using extremely high vacuums during filtrations (you can use a bleeder valve to control vacuum level very easily).
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
I use a plastic aspirator and can easily pull 25" @ 40PSI and 27" @ 70PSI
I also installed a 1-100PSI pressure gauge on the water line so that I can check the water main pressure and plan around its flux. Works fine for me.
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Thank you for the information. I'll buy an aspirator and use it with the garden hose. Would a water pressure of 30 PSI and a metal aspirator work? Or
would I need to buy a jet pump or plastic aspirator?
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
Does anyone know of a preparation of sulfur trioxide from chlorosulfonic acid? Industrially, chlorosulfonic acid is produced from HCl(g) and SO3.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
I've encountered someone on this site claiming that titanium dissolves better in 9% HCl then in 37%.
Is it true? Because I'm planning to dissolve some titanium flitter.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Maybe do to passivization? Here is a PDF. I just scanned over it but I think the temperature of the acid is key.
http://www.archivesmse.org/vol28_6/2866.pdf
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Holy crap.
Who knew that 30g of titanium flitter and 200 ml of HCl heated to about 80C will continue reacting due to the heat of the reaction. the house smells
of HCl. But at least I'm pleased.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Quote: Originally posted by Mixell  | Holy crap.
Who knew that 30g of titanium flitter and 200 ml of HCl heated to about 80C will continue reacting due to the heat of the reaction. the house smells
of HCl. But at least I'm pleased. |
lol, careful. HCl is persistent. *cough* My throat hurts just thinking about it.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Ok, the reaction is done.
But I got quite strange results:
The solution is quite concentrated and looks black, when a small amount is taken it looks very dark purple-brown.
But when I dilute it, the solution turns quite brown, resembling this shade: http://ontariohottubcovers.com/imgs/colors/color-cedar-brown... .
When I add hydrogen peroxide to it, it first turns slightly yellow (very transparent) and if I add more it turns blood red.
But what troubles me is it does not resemble the purple solution at all, as shown on Wikipedia: http://en.wikipedia.org/wiki/File:TiCl3.jpg .
What could it be (the titanium is 99.5% pure and the HCl is lab grade)?
Also, the solution turns blue on addition of concentrated HCl.
[Edited on 5-7-2011 by Mixell]
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Well, I figured it out, the brown color is due to Ti[IV] contamination.
And the blue color is due to the TiCl6 ion? Is it titanium in its III or IV oxidation state?
Is there any good use for this solution? Or I'll just leave in on my desktop and see how it turns clear?
Shame that it will go to waste.
|
|
|
sternman318
Hazard to Others
  
Posts: 121
Registered: 21-4-2011
Member Is Offline
Mood: No Mood
|
|
What does the squiggle that is bound the carbon represent?
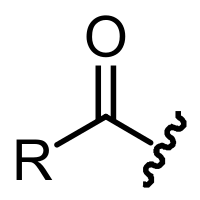
http://en.wikipedia.org/wiki/Acyl_group
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
That's just a shorthand to say that anything could be connected to the other end of the bond. Rather like an engineering drawing, if you were drawing
a closeup of the valve on the end of a pipe, you might make a wavy line across the end of your drawing of the pipe to show that the pipe continued
beyond the edge of the page but wasn't important to what you were trying to illustrate.
In organic chemistry it's usually used when your reactions are occuring at a specific group on a rather large structure, like cholesterol. Rather than
redrawing the entire cholesterol structure at each step of the reaction, you just draw the piece that is reacting in each step, and the reader can
assume that the rest of the molecule remains untouched.
In your specific case, only the part of the molecule you show is considered an "acyl group" - some sort of carbon chain (R) attached to a carbonyl
(C=O) attached to - something. An acyl group is just a fragment. If the "something" is an OH group, your acyl group is part of a carboxylic acid, if
an OR' group it is part of an ester, if an NHR' or NR'R" it is part of an amide, etc.
[Edited on 12-7-2011 by fledarmus]
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
What is the best way to dissolve nickel powder?
Concentrated sulfuric and nitric acid don't seem to do much, it reacts vigorously at first, but then the reaction stops.
[Edited on 13-7-2011 by Mixell]
|
|
|
sternman318
Hazard to Others
  
Posts: 121
Registered: 21-4-2011
Member Is Offline
Mood: No Mood
|
|
Thank you fledarmus!
And Mixell, have you tried diluting it? I have no idea if this is the case, but I know that concentrated nitric acid doesnt dissolve copper because it
forms a passivation layer. Diluting prevents the formation in that case.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
I tried diluting both of the acids, but nothing seem to work, may be I just didn't find that "magic concentration".
[Edited on 13-7-2011 by Mixell]
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
OK to boil sodium chlorate liquor electrolytically?
...instead of boiling after filtration? In other words, is there anything wrong with simply bumping the voltage/current up at the end of a run to boil
the liquor, then filter and proceed as usual (aside from a little extra anode erosion)? The solution is maintained at 80-85 degrees C anyway and
acidified throughout. I don't see how 2 or 3 extra amps would hurt but don't want to screw up this batch.
Thanks
|
|
|
jwarr
Hazard to Self
 
Posts: 85
Registered: 25-6-2009
Member Is Offline
Mood: No Mood
|
|
I'd like to remove carbonyl adulterants from my denatured ethanol. Would mixing it with sodium hydroxide and then distilling be sufficient?
|
|
|
jwarr
Hazard to Self
 
Posts: 85
Registered: 25-6-2009
Member Is Offline
Mood: No Mood
|
|
I'd like to remove carbonyl adulterants from my denatured ethanol. Would mixing it with sodium hydroxide and then distilling be sufficient?
|
|
|
| Pages:
1
..
9
10
11
12
13
..
30 |