| Pages:
1
..
9
10
11
12
13
..
28 |
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: |
What is the procedure benzyl bromide --> benzaldoxime? Do you have a ref? |
Well, I don't know about benzyl bromide --> benzaldoxime, the refs I found were for benzal bromide --> benzaldoxime, I think via in
situ hydrolysis to benzaldehyde. I wish it was benzyl, that would make life a lot easier...
Here:
http://www.wipo.int/pctdb/en/wo.jsp?IA=US1989002377&DISP...
Maybe one could brominate a benzyl bromide under more mild conditions?
Or maybe there's a different way to directly add -CH2-CHBr2 without fucking with the methoxys?
EDIT:
Maybe all hope is not lost. Apparently aryl alcohols can be converted to oximes. Looks interesting, since hopefully you could get the substituted
phenylethanols easily from hydrolysis of the haloethylation product.
Refs:
http://www.informaworld.com/smpp/content~content=a714009825~...
http://sciencelinks.jp/j-east/article/200201/000020020101A08...
Hmmm... There it may be possible to do an almost one-pot synth of benzaldoximes from benzyl halides. According to this benzyl halides can be hydrolyzed to alcohols at >95% yields, maybe one could take the crude organic layer and directly add CrO3/Al2O3 and
then hydroxylamine HCl to get the oxime. Don't know about yields on the second part though. Have to get that ref.
Edit2:
I see, now that I have the ref, it just is a one-pot benzyl alcohol-->benzaldehyde-->benzaldoxime, nothing very special. Still, it gets
surprisingly good yields..
If only you could easily get the 2-phenylethyl halide, everything else would be easy... Does anyone have some information pertaining to
haloethylation? Halomethylation seems to proceed in high yield, but haloethylation seems to be a different story... Not that I've found much
useful information on it. Maybe the aqueous/PTC type reaction that PainKilla posted could work with acetaldehyde?
[Edited on 29-11-2008 by 497]
[Edited on 29-11-2008 by 497]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
| Quote: | | Well, I don't know about benzyl bromide --> benzaldoxime, the refs I found were for benzal bromide --> benzaldoxime, I think via in situ
hydrolysis to benzaldehyde. I wish it was benzyl, that would make life a lot easier... |
I don't understand are you trying to make phenylmethylamines? Maybe I am just missing something.
| Quote: | | Does anyone have some information pertaining to haloethylation? |
Look for a ref posted by me, it is a review of halomethylation, that has a section about haloethylation/propylation. IIRC the products are
1-phenyl-1-halo-ethanes.
The quickest way to a phenylacetaldehyde that I can think of is via a fiedel-crafts with ethylene oxide followed by a careful oxidation of the
alcohol. Watch out though, ethylene oxide is a toxic carcinogen. Don't worry about the methoxies, they can take a beating before cleavage.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
497, from what I understand you are looking for ways to prepare benzyl amines and 1-phenylethylamines? At least that is what your not so
comprehensible posts indicate but then again in some other posts it appears you want to prepare 2-phenylethylamines. Please pay more attention to what
you are talking about as you tend to clutter this thread which is supposed to be for short questions only. If you want to continue like you do
now I suggest you to open a thread in Beginnings or Organic section.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: |
I don't understand are you trying to make phenylmethylamines? Maybe I am just missing something. |
I'm operating under the assumption that having an extra carbon (e.g. starting with 2-phenylethyl bromide) will react similarly.. Am I wrong here?
| Quote: |
Look for a ref posted by me, it is a review of halomethylation, that has a section about haloethylation/propylation. IIRC the products are
1-phenyl-1-halo-ethanes. |
Sad, you're right, just 1-phenyl-1-halos... too bad. It's funny, that ref has about the most information on haloethylation I've ever seen in one
place..
| Quote: |
The quickest way to a phenylacetaldehyde that I can think of is via a fiedel-crafts with ethylene oxide followed by a careful oxidation of the
alcohol. Watch out though, ethylene oxide is a toxic carcinogen. Don't worry about the methoxies, they can take a beating before cleavage.
|
Hmm speaking of ethylene oxide, I did see where you can react the grignard phenylmagnesium halide with ethylene oxide and then acidify to get
phenylethyl alcohol. Don't have too much experience/knowledge about grignards, would this be a useful route? Too bad having to deal with ethylene
oxide would make it not very practical..
@Nicodem- I'm only after 2-phenylethylamines, sorry for the confusing posts, a lot of them were written late at night when I was very tired.. I think
I'm pretty much done discussing the subject anyway.
[Edited on 1-12-2008 by 497]
[Edited on 1-12-2008 by 497]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, i think the major problem would be the ethylen oxide.. Making it and using it (safely)..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
If one wishes to synth ethyl benzoate from benzoic acid, would denatured ethanol (with methanol) work, or would i get by products. Or alternatively
does anyone have details of a source? I cannot seem to find high temp solvents such as ethyl benzoate, decalin or undecane. (uk). I imagine you'd get
ethy and methyl benzoate, both of which would be useful as high temp solvents?
[Edited on 1-12-2008 by Aubrey]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Just reflux your denaturated ethanol over some CaO and NaOH to dry it up to absolute and destroy most denaturants, fractionnate it (disgarding the
first 5% to remove the methanol-rich fraction), and directly use to esterify (using cat H2SO4, benzoic acid and excess EtOH).
You can even distill part of the excess ethanol during the reaction after a few hours reflux to remove the formed water via the 96% azeotrope, hence
the advantage of starting with absolute ethanol.
Better limit the amount of impurities, as I suppose you will not be fractionnating the ethyl benzoate, carbonyls in particulia can be problematic
during high temp rearrangement, aswell as amines, etc. even in catalytic amounts.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I am making copper sulfate by electrolysis of dilute sulfuric acid with two copper electrodes. I am using DC current (12 volts 1.7 amps.) One of the
electrodes corrodes and gives off blue color (copper sulfate) and the other bubbles and collects a spongy red black material which I am quite sure is
copper.
I know DC current is almost always used for electrolysis but could I use AC? Both electrodes are the same and I don't care which one makes copper
sulfate. If anything I would think I could conserve copper because it would go from electrode to electrode but I wanted to check first because my
knowledge of electrochemistry is very limited.
I am using a large transformer initially used to charge a laptop but it gets hot and progress seems to have slowed any advice? Should I add fresh
electrolyte?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by crazyboy
I know DC current is almost always used for electrolysis but could I use AC? Both electrodes are the same and I don't care which one makes copper
sulfate. |
But you do care which one makes copper, and neither will be making either if you run AC. The average is zero.
What you actually need is a seperated cell so that the copper ions aren't reduced by the cathode. They will naturally be drawn to the cathode
regardless, making direct electrolysis a poor method of production.
A neutral salt, however, will precipitate Cu(OH)2 (or if hot, CuO, or if Cu(I) is stable in solution (as in a strong NaCl solution), Cu2O), which
precipitates with no change to the electrolyte and can be dissolved in a seperate step.
| Quote: | | I am using a large transformer initially used to charge a laptop but it gets hot and progress seems to have slowed any advice? Should I add fresh
electrolyte? |
Do what huh??
Tim
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
what is SDA-2B ethanol?
Chemistry=Chem+ is+ Try
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Specially Denatured Alcohol Formula No. 2-B, denatured with rubber hydrocarbon solvent - hydrocarbons boiling between 80 and 120 C, mostly alkanes
(ligroin) but at one time including appreciable amounts of benzene and toluene.
A search on-line using your question would have given you that information much more quickly.
|
|
|
raiden
Harmless

Posts: 38
Registered: 4-2-2008
Member Is Offline
Mood: Curious
|
|
Do I have to use a special vacuum oil or is the one used in fridges fine yo?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yo??
Yes, vacuum oil is a specialty oil. You must use it.
Tim
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
what is schema of N-methyl-d,l-alanine and 2 methyl alanine
same?
[Edited on 5-12-2008 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
| Quote: | Originally posted by hector2000
what is schema of N-methyl-d,l-alanine and 2 methyl alanine
same?[Edited on 5-12-2008 by hector2000] |
No. 2-methylalanine (2-methyl-2-aminopropanoic acid) is achiral. N-methyl-d,l-alanine is the racemate of N-methyl-2-aminopropanoic acid.
2-methylalanine is on the left. both enantiomers of N-methyl-d,l-alanine are on the right.
[Edited on 12-5-08 by UnintentionalChaos]
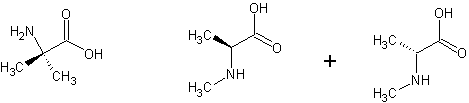
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
what is Eschweiler Clarke reaction
please say with real example with instruction
Chemistry=Chem+ is+ Try
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
The Eschweiler-Clarke reaction (also called the Eschweiler-Clarke methylation) is a chemical reaction whereby a primary (or secondary) amine is
methylated using excess formic acid and formaldehyde.[1][2][3][4] Reductive amination reactions such as this one will not produce quaternary ammonium
salts, but instead will stop at the tertiary amine stage. It is named for the German chemist Wilhelm Eschweiler (1860-1936) and the British chemist
Hans Thacher Clarke (1887-1972).
........source,
http://en.wikipedia.org/wiki/Eschweiler-Clarke_reaction
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
do you have any example with this reaction?
i want to use it for methylation amine but idont know the ratio of formic acid and formaldehyde and the temp and time.
if some one write a complete reaction of methylation any amine then will be very useful for me
thx
Chemistry=Chem+ is+ Try
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
There is a thread on methylation where several methods are presented , within each article there is always an experiment example with
ratios........also you should try the search function on this forum to see what's been posted... solo
https://sciencemadness.org/talk/viewthread.php?tid=8375&...
https://sciencemadness.org/talk/viewthread.php?tid=7512&...
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Has anyone seen anything about the industrial synthesis of piperonyl butoxide? Wiki gives safrole as being a precursor to it, which means they've
added the polyalkylether chain to the ring. I want to insert a substituted phenylacetyl moiety in the same spot piperonyl butoxide has the
polyalkylether chain and figured any info on the butoxide synthesis might help.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Piperonyl Butoxide, or 5-[2-(2-butoxyethoxy)ethoxymethyl]-6-propyl-1,3-benzodioxole, is used as a synergist in cheap aerosol insect killers, and
lice-killing preparations, which also contain at least one pyrethroid compound (which are compounds extracted from the African pyrethrum, a species of
chrysanthenum, and also occur in some other related daisy species, and synthetic variants of them, usually containing a 3-membered ring). However, it
is substantially less lethal to insects than pyrethroids, but much cheaper to make, having been made mainly from safrole since 1947.
The stuff is a potent cytochrome P450 inhibitor. This family of enzymes act as the principal detoxification pathway for many pesticides. Inhibiting
the detoxification pathway allows higher unmetabolised systemic concentrations of the active insecticide to remain within the target animal for a
longer period.
See:
http://en.wikipedia.org/wiki/Piperonyl_butoxide
http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC33...
http://www.safe2use.com/poisons-pesticides/inerts/piperonyl-...
http://www.the-piedpiper.co.uk/th13(p).htm
http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601105.htm...
http://npic.orst.edu/factsheets/pbogen.pdf
http://www.scorecard.org/chemical-profiles/summary.tcl?edf_s...
http://www.inchem.org/documents/iarc/vol30/piperonylbutoxide...
http://www.medicinenet.com/pyrethrins_and_piperonyl_butoxide...
http://www.chemindustry.com/chemnames/p/piperonyl_butoxide.h...
http://www.elsevier.com/wps/product/cws_home/700827 (review of book about it)
http://www.emea.europa.eu/pdfs/vet/mrls/053798en.pdf
http://www.drugs.com/mtm/piperonyl-butoxide-and-pyrethrins-t...
[Edited on 6-12-08 by JohnWW]
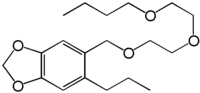
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
JohnWWW- basically the links to the first page of a google search? I wasn't looking for general information, but more importantly, what reaction is
used to go from safrole --> piperonyl butoxide. I suspect the safrole is hydrogenated first. I am hoping it is or is similar to an FC alkylation,
because that would make it very easy to add an acyl moiety instead.
The propyl group on the butoxide will be replaced by a protected 2-hydroxyethyl group.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
The merck index gives these two patents for the preparation. Also: Science 105, 530 (1947). 
Seems to use hydrogenation then chloromethylation of saffrole.
[Edited on 5-12-2008 by sonogashira]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Here are the details of the book that has been written about the stuff:
Piperonyl Butoxide - The Insecticide Synergist (Academic Press - 1998)
by Denys Glynne Jones, Glynne D. Jones, Denys Glynne Jones (Hardcover)
http://www.springerlink.com/index/Q54R2K270TU212N4.pdf (need paid subscription to access)
http://doi.wiley.com/10.1002/(SICI)1526-4998(200001)56:1%3C101::AID-PS91%3E3.3.CO;2-V
Piperonyl Butoxide contains 20 chapters contributed by world experts in the field on the properties, uses, plant metabolism, and mammalian and
environmental toxicology of piperonyl butoxide. The mode of action of piperonyl butoxide is discussed as well as many other specialist topics,
including the measurement of synergism in the laboratory, and the potential use of this chemical alone for the control of whiteflies, as well as with
insect growth regulators.
This book will prove to be a valuable reference for all concerned with the designing of safe and cost-effective insecticide formulations, particularly
those used in the home, industry, or on or near animals and food.
Found on Gigapedia at: http://gigapedia.org/items/199506/piperonyl-butoxide
The Rapidshare download link has been posted in the References section, under Organic Chemistry books.
[Edited on 6-12-08 by JohnWW]

|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
i have 2 question:
1-does norephedrine will methylate by aluminum amalgam+formaldehyde+Etoh--->Ephedrine or psudoephedrine?
2-in the industry for make large scale of psudoephderine use of lpac and then reduce lpac to psudoephedrine but making lpac need pyruvic acid that is
expensive.i wonder if in the industry use pyruvic acid how the price of psudoehedrine is cheap!!
[Edited on 6-12-2008 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
| Pages:
1
..
9
10
11
12
13
..
28 |