| Pages:
1
..
9
10
11
12
13
..
25 |
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
First make the solution alkaline, producing free hydrazine, and next add a mild oxidising agent.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A little request about attachments, if you will make them about 500 pixels maximum width .....oh never mind ...
what do I know.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
US Patant 7118655 - Direct synthesis of hydrazine through nitrogen fixation by means of two-photon absorptions
------------------------------------------------------------------------------------------------------------------------------------------------
http://www.patentstorm.us/patents/pdfs/patent_id/7118655.htm...
------------------------------------------------------------------------------------------------------------------------------------------------
Question: Since the main part of this relys on the Excition and disassociation or Nitrogen molecule would it be possible for a Transversaly excited
Nitrogen laser to replicate these conditions if hydrogen was pumped in at A two times molar ratio?
TE nitrogen lasers rely on a super fast so called blumenthal circuit that can produce pulses on the range of 2.5ns - < 1ns.
There output is in the range of 337.5nm. I feel that even the high power per pulse and frequency may still me in effective though but I was woundering
if anyone with more knowlage on this has anyideas that could make this patant in the range of amateur abilitys. N2 TEA laser are not hard to make just
finiky but if I can make one anyone can.
Any one have any thoughts on this?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
A couple of articles. Rather than take a chance on an archive, which this board does not like, or make two posts, I'll combine the two.
In JACS 51, 265 (1929), there is the preparation and concentration of hydrazine hydrate solutions by azeotropic removal of water with xylene and
toluene. They seem to say that the methods given in Vanino for hydrazine hydrate (pp. 141-2 of the scanned ed.) are lower yielding, and that it is
better to make a dilute solution and concentrate it. It would be nice to read the Adams reference that they cite at the beginning, but the only
available edition AFAIK of OCR so far (from 1919) does not mention hydrazine.
In JACS 76, 3914 (1954), there are a few experimentals on hydrazine from the mono or dihydrochloride. They used a few methods here.
A sodium methoxide route.
NaOH, BaO, CaO.
And they mention that they had high-yielding experiences using cyclohexylamine, among the high-boiling amines given in US2537791
Attachment: hydrazine.pdf (741kB)
This file has been downloaded 2095 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sedit
US Patant 7118655 - Direct synthesis of hydrazine through nitrogen fixation by means of two-photon absorptions
Question: Since the main part of this relys on the Excition and disassociation or Nitrogen molecule would it be possible for a Transversaly excited
Nitrogen laser to replicate these conditions if hydrogen was pumped in at A two times molar ratio?
... |
The N2 is only being excited, not disassociated. Quoting from that patent:
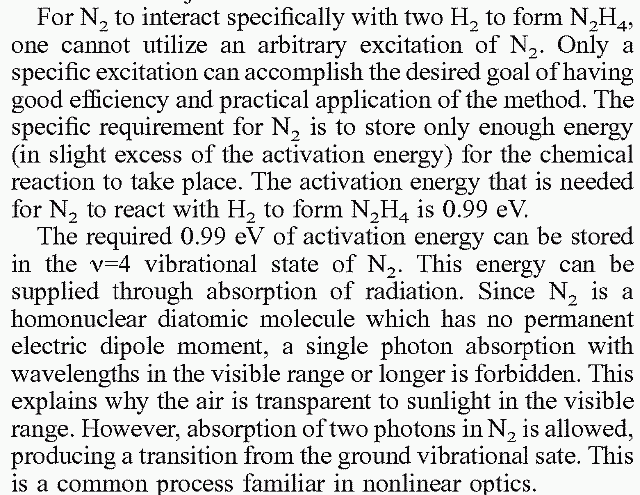
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by not_important
The N2 is only being excited, not disassociated. Quoting from that patent: |
This is definitely
industrial-grade. The cross section for two-photon absorption is rather small at low photon densities, so that means high densities, which means high
luminance and everything that entails. Even with high fluences, the rate of absorption is fairly small in terms of transmission percentages, so you
want to reuse the beam path as much as possible. Thus a reasonably efficient machine needs to be pretty big for its unit energy consumption to be
reasonable.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I ran the hydrazine sulfate synthesis described on Sciencemadness that uses 10% NaOCl solution and urea, last year. This synthesis worked well and I
obtained 185g of hydrazine sulfate. I am about to run the synthesis on a 1/2 scale in a 3 liter flask with mechanical stirring. The foaming problem
seems to be the only issue with this synthesis; the first batch I attempted overflowed the reaction flask. At least it was in a fume hood, so the
clean up was fairly easy. This was with the full scale procedure in the 3L RBF. I had the plastic funnel attached to the flask as stated. The second
attempt was conducted in a 5 gallon plastic bucket and transferred to the 3L RBF after the foaming stage via a syphon. I obtained a good yield of
hydrazine sulfate after neutralization, 185g. I am trying to see if this reaction could be scaled down without severly decreasing the yield. I
synthesised 2,4 dinitrophenyl hydrazine, and 3,5-dimethyl pyrazole using some of the hydrazine sulfate. I plan to synthesize some luminol using
readily available chemicals, including the hydrazine sulfate produced via this reaction.
Amateur NMR spectroscopist
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Good work. 185g does seem like a rather large batch. But I suppose that all depends on the intended purpose. Perhaps the originator was using it
for pyro or rocketry?
I also want to make luminol and the 2,4 dinitrophenylhydrazine. It seems your wish list is very similar to mine. 
Was this the synthesis from Mr Anonymous?
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
The synthesis that I conducted was a full scale Mr. Anonynmous synthesis. I ran a 1/2 scale synthesis today. I used a 3 liter 4 neck flask for the
synthesis, along with a mechanical stirrer. The 3 liter flask could barely contain the foam. Lots of crystals have formed; I still have to filter and
wash the product.
Amateur NMR spectroscopist
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I hate to bring up an old threed to ask nothing more then a question and not give anything in return but does anyone have information of the method
briefly mentioned about the production of hydrazine thru the reduction of nitrates or nitrites using Zinc in a neutral solution?
He sites Ber. 21, 2637 (1888) as a reference but I do not really know how to find this paper thru this information alone.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Mr Anonymous method & ideas 4 improvement
I have performed the hydrazine synthesis according to Mr. anonymous method numerous times now and it worked well. I even scaled the quantities so I
could use ordinary battery acid instead of precious conc. H2SO4. The NaOH sol. needs to be more conc. in this case to compensate for the extra H2O
which will cause NaCl to precipate while Cl2 is bubbled in. It makes no difference in the end tough coz total amount of water is the same. You just
need one more boiling to get everything into solution after it is neutralized with HCl/H2SO4. The hydrazine sulfate will then start to precipate.
I think this is an almost perfect method for OTC hydrazine production. All the chemicals are easy to come by - battery acid, NaOH, TCCS, HCl for Cl2
production and urea which is a cheap fertilizer at least here where I live.
However quite a lot of hydrazine sulfate is lost due to its considerable solubility and the large amounts of H2O required to keep all the reaction
products in solution.
I tought about ways to overcome this problem. Obviously what is needed is a less soluble hydrazine salt. Sulfite and oxalate pop to mind as
alternative anions but I found no solubility info.
However what I do know is that hydrazine forms insoluble complex salts with certain metals. Both nickel nitrate and sulfate form an almost insoluble
precipate upon addition of hydrazine.
There was brief mention in this thread of a "copper hydrazine sulfate". I was unsure if hydrazine sulfate or hydrazine alone was coordinated to the
copper in this case, so I tried adding CuSO4 to a dilute solution of hydrazine sulfate. No precipation was observed so I'm inclined to think that the
mentioned complex is actually [Cu(N2H4)2]SO4.
The idea is to obtain *all* the hydrazine by precipation as an insoluble complex salt, and then recover it from that salt.
It might be as easy as following Mr. anonymous' instruction up to the point where a dilute hydrazine solution is obtained. This solution must then be
neutralized to get rid of CO3(2-) otherwise insoluble metal carbonates will be precipated during the following step. To this neutralized solution is
then added a conc. solution of any soluble Ni or Cu salt, and pretty much *all* hydrazine should be precipated due to complexation. I think that the 2
non-binding electrons in N2H4 can bridge two metal ions leading to a polymer-like structure which is insoluble in water. This should work with
Ni,Cu,Zn,Co and maybe others as well.
The question now is: How could the hydrazine be recovered from such a coordination compound?
Well here's an idea: Cu(OH)2 decomposes to CuO at 80°C. This should set the coordinated N2H4 free because there is covalent bonding in CuO. Thus
destillation of [Cu(N2H4)2]SO4 with NaOH and a small amount of water might to the trick. It needs to be done in a copper vessel (a bent copper pipe
might do well) because hydrazine and NaOH eat thru glass easily at elevated temperature.
Obviously this should not be tried with copper salts that have oxidizing anions. Copper sulfate should be fine.
Of course the above mentioned method could also be used to obtain [Ni(N2H4)3](NO3)2 from a neutralized dilute hydrazine solution. After all its a
powerful primary 
[Edited on 11-8-2009 by Taoiseach]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Try recovering the hydrazine as ketazine.
Make the solution alkaline, add acetone, stir and wait a while, then extract with toluene.
Reflux the toluene phase with dilute H2SO4 under stirring.
If hydrazine sulphate crystallizes out upon cooling, you have won.
I think you should try the extraction out first with a hydrazine sulphate solution to which 2 mol NaOH have been added, so you don't have any
interferences from byproducts from the synthesis.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
There is a problem with the suggested method, which uses copper. Copper(II) ions are reduced by hydrazine and this reaction is quick. I once did the
experiment of making a copper(II) hydrazine complex in somewhat alkaline solution. The only thing I obtained was a red/brown precipitate, which either
is Cu2O, or maybe even metallic copper. The hydrazinium ion does not quickly reduce copper(II) ions. Reduction only occurs at neutral or alkaline pH.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quite a ways back in this thread, I believe Rosco posted a paper on monohydrazinium cyanurate, which is weakly soluble at best. It also details that a
column of Cyanuric acid granules can be used to recover almost all the hydrazine from even dilute solutions.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Sounds good. Isocyanuric acid is a beproduct of chlorine production via TCCS+HCl. Question is: How can hydrazine be recovered from the cyanurate?
If I remember correctly, Na cyanurate is well soluble but the Ca salt is insoluble. Reaction of hydrazine cyanurate with CaCl2 might then give
hydrazine hydrochloride plus Ca cyanurate.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Sounds good =
http://www.youtube.com/watch?v=leEO7_BV590&fmt=18 First Light
Answer = Add heat 
More good sound (with added heat ) =
http://www.youtube.com/watch?v=9OJgdlaRzu4&fmt=18
All I Want ....... 
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
wondering, could this be done anywhere within economical reasons.
I know there are by far better methods concerning money still this would be very easy and time saving.
Hydrazine is very easily to destroy by heat right?
So would very little heat be enough to sweat the hydrazine.
Else i can imagine most of it would undergo themolysis/destruction.
What a fine day for chemistry this is.
|
|
|
uchiacon
Hazard to Self
 
Posts: 87
Registered: 3-7-2009
Location: The Jungle(neezeelund)
Member Is Offline
Mood: Financial
|
|
So I just had a flick through this thread, but I didn't see much discussed on the synthesis of hydrazine hydrate. There were a few suggestions about
hydrazine sulfate and NaOH in silver coated glassware, but there wasn't really a proper synth.
I would be using it for the synthesis of sodium azide.
Could anyone please direct me to one?
And I was also just contemplating whether or not I should just buy hydrazine hydrate; it comes in at about $50NZ for 250g of it. $50 NZ is about
$40US.
What do you guys think? Is the synth worth the effort?
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
You dont need silver, copper will do fine.
|
|
|
uchiacon
Hazard to Self
 
Posts: 87
Registered: 3-7-2009
Location: The Jungle(neezeelund)
Member Is Offline
Mood: Financial
|
|
Yes, but even so, it will render my whole distillation kit unsuitable for pretty much anything else, and I only really use it for distillation of
nitric acid.
Is it possible to make a weaker hydrazine hydrate solution with just NaOH,water and Hydrazine sulfate and use that for sodium azide? Or wont it work?
How would you coat the whole inside of the glass, would you only need to coat the boiling flask, and why do you need to coat it in the first place? Is
hydrazine corrosive to glass?
[Edited on 04-07-09 by uchiacon]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
You didn't look very hard.
|
|
|
Myfanwy
Hazard to Self
 
Posts: 68
Registered: 20-10-2009
Member Is Offline
Mood: No Mood
|
|
Yesterday i tried to make hydrazine sulfate of Lye, sulfuric acid and Chlorourea (is that the english name of chlorharnstoff?), without Gelatin to
bound the Metallions. I always used distilled water, and cleaned my glassed, but unforntunately no N2H6SO4 crystallised in the cold 
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Myfanwy  | | Yesterday i tried to make hydrazine sulfate of Lye, sulfuric acid and Chlorourea (is that the english name of chlorharnstoff?),
|
The English name of the compound in question is N,N'-Dichlorourea (Ger., N.N'-Dichlor-harnstoff), or dichlorourea. There are several N-chlorinated
ureas though.
| Quote: | | without Gelatin to bound the Metallions. |
Gelatin is not needed, a variety of other compounds work to bind metal ions (in addition to this thread, also read the patents by Raschig).
| Quote: | I always used distilled water, and cleaned my glassed, but unforntunately no N2H6SO4 crystallised in the cold  |
Your explanation is not clear on what you did. Follow closely what has been done and works. Dichlorourea is not without hazard. Remember that if you
chlorinate too much, you form extremely dangerous NCl3. If you warm it up in water, NCl3 forms. Not things to take lightly.
|
|
|
Myfanwy
Hazard to Self
 
Posts: 68
Registered: 20-10-2009
Member Is Offline
Mood: No Mood
|
|
No i mean Monochlorourea - CONH2NHCl.
I cant find any information about this compund.
Okay i bubbled 18,3g Cl2 into a solution contaning 15,5g CO(NH2)2.
then i cooled the Sodium hydroxide solution(31g in 60g water) and the monochlorourea solution in an icebath.
after adding both together it gets warm (from 8°C-30°C).
Then i added about 50g 50% H2SO4.
CONH2NHCl + 3NaOH -> N2H5OH + Na2CO3 + NaCl
i think i tooked too much water and cooled not efficiently.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Quote: Originally posted by benzylchloride1  | | I ran the hydrazine sulfate synthesis described on Sciencemadness that uses 10% NaOCl solution and urea, last year. This synthesis worked well and I
obtained 185g of hydrazine sulfate. I am about to run the synthesis on a 1/2 scale in a 3 liter flask with mechanical stirring. The foaming problem
seems to be the only issue with this synthesis; the first batch I attempted overflowed the reaction flask. At least it was in a fume hood, so the
clean up was fairly easy. This was with the full scale procedure in the 3L RBF. I had the plastic funnel attached to the flask as stated. The second
attempt was conducted in a 5 gallon plastic bucket and transferred to the 3L RBF after the foaming stage via a syphon. I obtained a good yield of
hydrazine sulfate after neutralization, 185g. I am trying to see if this reaction could be scaled down without severly decreasing the yield. I
synthesised 2,4 dinitrophenyl hydrazine, and 3,5-dimethyl pyrazole using some of the hydrazine sulfate. I plan to synthesize some luminol using
readily available chemicals, including the hydrazine sulfate produced via this reaction. |
The trouble with that synthesis, is that despite a great deal of detail its rather imprecise in its key point. For instance the volume of bleach is
measured with great precision 1892ml, but then the bleach strength is quoted at 10%. Just a 1% change to 11% bleach strength alters the water content
by 180ml, which makes a mockery not only of measuring the volume to that precision, but also the subsequent attempts to dissolve the NaOH in bleach,
and the urea, and gelatine in a barely adequate amount of water.
There is the possibility that what seems to be a round figure of 10% in reality is accurately measured. That is unlikely, as the author does not seem
to have performed bleach titrations. For instance he states that 10% bleach solutions decompose within a few weeks, to a couple of months. I have
done bleach titrations, and a solution 11.5% strong, is still 8.2% even after 3 months non air-conditioned storage in temperatures which have exceeded
40 degrees for long periods of time.
|
|
|
| Pages:
1
..
9
10
11
12
13
..
25 |