| Pages:
1
..
8
9
10
11
12
..
18 |
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This is very interesting. I will keep my eyes open and see if I can find anything pertaining to the subject. I didn't really look much farther than
the alcohol methods when I first learned to make azides. I was very happy and contented when I learned how to make it work. Though a large amount of
costly alcohol is used those methods are very reliable and robust, solidly proven as you have described.
I will keep my eyes open for alternative methods. The azides really are so many times better than the other primaries I was experimenting with prior
to.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yes azides are definitely a subject of interest for which a good practical working knowledge is a priority for anyone interested in the scientific art
associated with initiating explosives. It could be prioritized as "Explosives 101" kind of fundamental must have knowledge buried and hidden among
that voluminous work they would not dare to teach in college  , a deficiency
and oversight which any aspiring "blaster" would then seek to remedy as knowledge gotten from "independent further research". Of course that would be
the point to not "spoon feed" every secret thing to the student, but only sufficient knowledge to operate as a provocation to the student who must
then solve any remaining mystery for themself. We are taught only to a point, and thereafter we are self-taught and this is probably by design. The
impatient to know would criticize the design and question its necessity, question the teachers, question the intelligence of the design. It is what
it is. , a deficiency
and oversight which any aspiring "blaster" would then seek to remedy as knowledge gotten from "independent further research". Of course that would be
the point to not "spoon feed" every secret thing to the student, but only sufficient knowledge to operate as a provocation to the student who must
then solve any remaining mystery for themself. We are taught only to a point, and thereafter we are self-taught and this is probably by design. The
impatient to know would criticize the design and question its necessity, question the teachers, question the intelligence of the design. It is what
it is.
If we stick with the well proven non-aqueous methods there would still seem to be improvements possible to be made in the manipulations. My personal
impression is that a scheme which uses the dihydrazine sulfate prepared in advance as the material which will be freebased into alcohol, very likely
would represent such an improvement. Isopropyl alcohol is a good choice for the alcohol as described for the method of microtek and its variations.
There possibly could be advantage for use of different organic nitrites other than isopropyl nitrite, or maybe not. Surveying the possibilities there
are other organic nitrites which may have lower vapor pressure and possibly adequate stability, and which will either react directly with the
hydrazine in isopropanol or will transesterfy in situ to the isopropyl nitrite which will allow the desired reacton to proceed. Depending upon
availability other alternative solvent alcohols are also workable. So it appears that the "alcohol solvent method" is general and may be subject to
variations at the technicians discretion which may serve as refinement applied to the same basic method.
Ultimately there will be found several slightly different but still related methods all of which are workable with each approach having its advantages
or disadvantages compared with another variation. What will ultimately prove to be the "best" all around combination of solvent alcohol, organic
nitrite ester, and base, and hydrazine salt precursor material, is left to be sorted out with further experiments, while encouraged to know that
decent yields are probable to be gotten from any of the variations, as whatever variations are tested to try to optimize the particular combination.
There is plenty there to digest when surveying the "process chemistry" involved, plenty of food for thought there for the hungry mind of the curious
experimenter.
[Edited on 1-4-2013 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This is what I had of Microtek’s; there may be other posts that I am unaware of. Keep in mind these posts are almost 10 years old.
From E&W
"Microtek
September 9th, 2004, 12:00 PM
For sodium azide production I use a method which gives hydrazine in alcohol (from hydrazine sulfate) with little or no water:
- 1 mol dry HS is placed in a flask along with a suitable amount of anhydrous isopropanol.
- 1 mol of NaOH pellets are added and the contents are triturated with a glass rod until they begin to react, forming a slurry of hydrazine hydrate
and NaHSO4. This doesn't mix with the iPrOH, but forms a sticky goo on the glass.
- Another 1 mol NaOH is added which converts the NaHSO4 to Na2SO4 ( and forms a mol of water ) which separates cleanly as a white powder.
I would think that the Na2SO4 is a good enough dessicant to dry out the solution which can then be decanted.
- Another extraction or two with dry iPrOH recovers most of the hydrazine.
This alcoholic solution of hydrazine works well for producing sodium azide with isopropyl nitrite."
I also have this from E&W (2005), part 2 of his azo-clathrate synthesis.
"2) Production of hydrazine hydrate: 13 g hydrazine sulfate is placed in a suitable vessel along with 15 mL IPA, 99%. 4 g solid NaOH is added and the
pellets are crushed and triturated with the HS. After a few minutes, the powdered reactants will attain the appearance of a paste as the produced
NaHSO4 stubbornly holds on to the produced hydrazine hydrate. The paste is worked through a little more to ensure complete reaction, and then another
4-5 g solid NaOH is added and worked into the paste to convert the NaHSO4 into Na2SO4 which separates cleanly as a crisply dry powder.
The IPA solution of hydrazine hydrate is removed by decatation or with a syringe, and
the remaining powder is extracted a further two times with 10 mL IPA.
The advantage of using only IPA is that there is no possibility of cross-esterification during the reaction of isopropyl nitrite with hydrazine
hydrate, so there is no need to carry out the operation under pressure. Also, the practically anhydrous conditions causes the sodium azide to
precipitate completely and instantaneously from the reaction mixture as the nitrite is added."
A lot of what he said was very helpful, but what I also found was that I had to use a lot more isopropyl alcohol in order to dissolve the amount of
sodium hydroxide necessary to balance the reaction equation. I also found that the product was not produced immediately, but took half an hour or more
at room temperature before much of any product was seen and then 2 or more days to approach completion. He may have done many things differently to
control the reaction conditions, but I have not seen those posts. When operating at room temperature, I found using KOH to be a big improvement.
I think this part is mine, though I am sure somebody somewhere has done it before.
It may seem like a simple modification (and it is), but using potassium hydroxide in place of sodium hydroxide when using isopropyl alcohol as the
solvent provides a huge economy in alcohol (since KOH is much more soluble than NaOH in isopropyl alcohol) and time (since the reactants are at a much
higher initial concentration).
You can lead a horse to water, but you can't make him drink (I'll give you that). Not all knowledgeable people are generous with their knowledge
either though. Like you say these things are probably natural, even with large amounts of spoon feeding generally people need to work with the
material in order to really grasp what is going on.
[Edited on 31-3-2013 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
A passing thought I had
Ammonium Nitrate is decomposed by Hydrazine , replacing and liberating
Ammonia to form Hydrazinium Nitrate
NH4NO3 + N2H4 => NH3 + N2H4•HNO3
so what about Ammonium Nitrite to form Hydrazinium Azide.
Nitrite will oxidize Hydrazine just the same as it does Urea.
NH4NO2 + 2 N2H4 => NH3 + N2H4 + N2H4•HNO2 => NH4OH + H2O + N2H4•HN3
on Ammonium Nitrite
www.sciencemadness.org/talk/viewthread.php?tid=23807
www.sciencemadness.org/talk/viewthread.php?tid=6650
______________________________________________
Upon further reflection ( and meta search ) it appears things are
not as straight forward as one can hope for. According to The
Encyclopedia of Explosives & Related Items - PATR 2700
Hydrazinium Nitrite is a known stable salt , making it's further
transition to azide dubious.
H 196
Hydrazine (or Hydrazinium) Nitrite. N2H4•HNO2 ;
mw 79.06, N 53.15%; decomp or explodes on rapid heating;
colorless to yellowish hygr solid; sol in w & alc; insol in eth;
may be prepd by mixing solns of barium nitrite and neutral
hydrazine sulfate, as described in Mellor (Ref 1).
Explodes violently on impact and less so when rapidly heated.
When heated slowly it decomposes according to the equation:
N2H4•HNO2 => NH3 + N20 + H20
and this decompn is greatly accelerated by nitrous acid.
Refs:
1) Mellor 8, 472-3 (1946)
2) F.Sommer, ZAnorgChem 83, 119 (1913)
3) Clark, Hydrazine (1953), p 6
the often cited reaction of hydrazine with nitrite to form azide is
also clearly stated
H 192
Hydrazine is used for the preparation of hydrazoic acid according to:
N2H4 + HNO2 => HN3 + 2H20
or for the preparation of sodium azide (Ref 14).
14) Ulmann, 6, 206, Encyklopädie der Technischen Chemie (1951)
the essence of what I propose above is clearly mentioned also
A 606
pure NaN3, made from N2H4 and NH4NO2
(Ref 54) J.Nelles, Ber 65B, 1345-7 (1932) & CA
as is the reaction analogous to hydrazine with ammonium nitrate
A 537 - Hydrazinium Azide
prepd by pouring hydrazine hydrate over ammonium azide
and evapg the mixt in a flat dish placed in a desiccator. This
latter method of prepn was patented by Miller in 1936 (Ref 10).
10) E.Müller, GerP 634688 (1936) & CA 31, 511 (1937)
so what is one to think, experimentation will tell.
____________________________________
If someone is wondering about that apparent nonsequitor referring to
Ethylene Glycol Dinitrite posted by Rosco Bodine below, see _
www.sciencemadness.org/talk/viewthread.php?tid=6395&goto...
.
[Edited on 1-4-2013 by franklyn]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Rosco Bodine  | | If we stick with the well proven non-aqueous methods there would still seem to be improvements possible to be made in the manipulations. My personal
impression is that a scheme which uses the dihydrazine sulfate prepared in advance as the material which will be freebased into alcohol, very likely
would represent such an improvement. |
Hmmm, why didn't I think of that? I've caught myself reinventing the wheel while reviewing the matter. The original patents regarding freebasing
hydrazine into alcohol were in fact done using dihydrazine sulfate, which would simplify things as I have been thinking. So the freebasing done from
the monohydrazine sulfate has been taking a short cut, which causes twice the byproduct water from neutralization and increases the amount of alcohol
being needed for the extraction.
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
These are some pertinent references
GB900397 Freebasing hydrazine in ethanol
GB876038 Frebasing hydrazine in methanol
US3015542 US issue of GB900397
US2166698 ethylene glycol dinitrite
Attachment: GB900397 hydrazine extract using ethanol.pdf (284kB)
This file has been downloaded 935 times
Attachment: GB876038 Hydrazine extract in alcohol.pdf (249kB)
This file has been downloaded 1139 times
Attachment: US3015542 Hydrazine in Ethanol from dhydrazine sulfate.pdf (113kB)
This file has been downloaded 2317 times
Attachment: US2166698 ethylene glycol dinitrite and glycerin nitrite esters.pdf (175kB)
This file has been downloaded 1044 times
[Edited on 1-4-2013 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I remember you bringing up dihydrazine sulfate a year and a half ago or more. It is a good idea which reduces the amount of water produced by half. I
am not quite sure why more water would require that there to be more alcohol used for extraction. I thought the water was a problem because it caused
more of the sodium or potassium azide product to be dissolved or lost in the reaction mixture.
There seemed to be an indication from some of the earlier posts that the sodium sulfate produced during freebasing may be absorbing much of the water
produced, but I don't know. The idea of using desiccants might be a good one.
Description of Drierite taken from http://www.chem.ucla.edu/~bacher/Specialtopics/Drying%20Agen...
Calcium sulfate (n=0.5, e=0.004 mg/L) is a neutral and good drying agent. However, it does not have a high capacity, which makes it useless for very
wet solutions. The commercially available Drierite contains cobalt chloride as indicator (dry: blue, wet: pink), which can be leached out into various
solvents i.e. ethanol, DMSO, DMF, ethers, etc. Drierite is often used in desiccators. If the compound is pink, the water can be removed by heating the
compound to 210 oC for an hour.
The text "Purification of Laboratory Chemicals" says isopropyl alcohol can be dried by adding CaSO4.
Could calcium sulfate or some other desiccant be added before or during the extraction process to remove water?
I know Rosco mentioned Glauberite [Na2Ca(SO4)2] in some previous posts.
[Edited on 1-4-2013 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I was having a little fun earlier with some folks here, probably with Len in mind more than others, sorry I couldn't resist 
Look again at the titling on that post paragraph linked above usual behavior of hydrated salt does not apply and you will understand
why it would be of limited usefulness to try to use any ordinary dessicants to try to dry an alcohol solution of hydrazine hydrate. The hydrazine
hydrate tenaciously holds onto water and wins the tug of war for the water unless some special approach is used like distillation fractionation or
azeotroping a solvent / water mixture, or in the alternative to use chemically reactive schemes like an alcoholate such as sodium ethoxide or sodium
methoxide as a part of the neutralization scheme in place of simply using a base like NaOH simply dissolved in the alcohol.
The speculation which I was making about Glauberite was more intended towards a cleaner precipitation of the sulfate byproduct of the freebasing
having a more compact crystalline form, than was it meant to address at all the byproduct water, which Glauberite would not do anyway since Galuberite
is an anhydrous double salt. The Glauberite would likely have its greatest utility in the prelimary conversion of the monohydrazine sulfate to
dihydrazine sulfate where the dihydrazine has such great solubility in water, and before an alcohol system is later used for the freebasing scheme
applied to a previously isolated and dried dihydrazine sulfate. It is possible the Glauberite may have utility also in the freebasing scheme, but it
would not be any dehydrating property that is the reason. The lowered solids content would make for easier physical manipulation to stir a slurry of
sulfate byproduct, and by working with dihydrazine sulfate there will only be half the bulk of byproduct sulfate, as well as half the byproduct water
in the freebasing and extraction stage.
The added quantity of alcohol for the more shortcut scheme is simply the relative amount of alcohol being greater to have an end resulting solution
having a comparable concentration of water. Twice as much alcohol is required for the shortcut scheme (at least) and maybe even a little more in
comparison to the proposed scheme which involves an easier manipulation for physical reasons.
If an alcoholate is contemplated being used also as a chemically reactive dehydration, there is benefit there also because only half as much byproduct
water is encountered when freebasing the dihydrazine sulfate as is gotten by the "one pot" shortcut method. For a bulk synthesis expecially where a
larger amount of material will be made, these refinements would be of greater concern with regards to the efficiency of the process. For small scale
syntheses, the simplicity of the "short cut" method makes waste of solvent or inefficiency less concern, but for any scaling up of the process, the
usefulness of the refinements would become apparent. Since I tend to work medium scale then I am more aware of these kinds of "process" efficiencies,
which could also be applied to the smaller scale if it was estimated to be worth the extra effort on the smaller scale.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Ok, now that I am looking at the reaction equations I see that all the water produced is bound to the hydrazine forming hydrazine hydrate. For some
reason I was thinking of unbound water (it has been over a year since I really looked at this at all, and I'm not really a chemist).
When using isopropanol the amount of alcohol used is dictated by its rather low ability to dissolve the hydroxide. The saving grace seems to be that
the azide products also seem to be very insoluble in isopropanol.
Yeah, efficiency really isn't a great big concern for me because I am usually only making a few grams for some caps. When you are only using 0.3g per
cap, a few grams can go quite a long way. If it costs me an extra buck or something to make a small batch it is no big deal, especially if it saves me
time and effort. I can see how my methods would be very uneconomical large scale though.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
To expound upon what I am trying to communicate it would be good to see the following post in the dedicated hydrazine thread. It further describes
what is contemplated as a method for virtually anhydrous hydrazine in isopropanol possible to be gotten by freebasing dihydrazine sulfate using
aluminum isopropoxide in isopropanol. I do not know if this has ever been done. But it appears to be the logical progression that such a proposed
route is possible.
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
As an aside there is also some modification of the hydrazine synthesis reportedly using manganous sulfate as a catalyst. The "Russian method"
from a Russian textbook Practical_Inorganic_Chemistry.Vorobyova.O.I_Dunaeva.K.M_Ippolitova.E.A_Tamm.N.S.1987
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
There is a patent reported US3227753 Biurea or Hydrazine from Hypochlorite and Urea which describes a similar process so this may be of interest as a
possible further refinement on the hypochlorite urea process.
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
Scale absolutely has bearing on the choice of methods used for convenience. Shortcuts are allowed for small scale work, and I never really explained
before that was what was deliberately done to make a "one pot" method from a generally more complicated industrial process. It is good to know this
however, because it can apply if any scaleup is contemplated or if problems are encountered with the small scale "one pot" methods, then those
problems may be resolved by the longer route or other measures done to refine the process.
Brainstorming is allowed also. For example I just thought of the possible refinement of using aluminum isopropoxide in ethanol, denatured alcohol to
do the freebasing of dihydrazine sulfate, resulting in anhydrous hydrazine in ethanol with a small percentage of byproduct isopropanol, a solvent
system being mostly ethanol in which the NaOH would be highly soluble. Then for the nitrosation a low volatility nitrite like diethylene glycol
dinitrite, or alternately butyl nitrite or amyl nitrite, or if stability of glyceryl di or trinitrite is sufficient, then the glyceryl nitrite could
be used.
Such a scheme could lead to a quantitative yield for the process regardless of scale.
So now you know where "Mr. Anonymous" was going with all of this that took ten years to get said ....connecting all the dots. Dots are hereby
declared "connected". 
I really wanted to get all these details down before the next forum backup so the communication transmission would be complete. I'm sure there was an
article or two missed along the way, but that is pretty much all I've got on this to this point, for the "hydrazine chronicles" or Martian (lander)
chronicles, or azide / tetrazole chronicles ....whatever.  It has been a long
ride, but a lot of fun sharing. It has been a long
ride, but a lot of fun sharing.
[Edited on 1-4-2013 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for sharing. Contributors like you, Microtek and some others have definitely helped me develop some of the skills I now possess.
As Microtek originally said isopropanol is good in the sense that there is no possibility of cross esterification. What I need to figure out is a way
to add the reactants (or at least the sodium/potassium hydroxide) to the reaction mixture, in the isopropanol solvent, in small increments instead of
all at once. If I could do that the process would require a lot less alcohol. I suppose at the scale that I am working at this sort of process, with
increased complexity, is probably not going to be much of an advantage all costs including time considered.
[Edited on 2-4-2013 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
If anybody had told me that an anonymous post here would lead to a ten year incremental tour of the literature with review of experiments and proposed
experiments involving hydrazine and related compounds, I would have chuckled and said sure, 10 years huh, you gotta be kidding me. But truth is
stranger than fiction. This has got to be an internet discussion forum record of some kind. This azide thread should get a sticky IMO. It seems to be
the main thread for azides, and along with tetrazoles seems to be a natural related interest energetic material for the general topic of hydrazine.
The process refinement priority for the alcohol reaction system which seems most promising would be preparing and isolating the dry dihdrazine sulfate
in advance as the precursor for freebasing.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have been wondering for a long time why this thread was not stickied.
Since brainstorming is allowed, I have another idea.
Notes on the Preparation of Absolute Isopropanol
Lewis Gilson
J. Am. Chem. Soc., 1932, 54 (4), pp 1445–1445
Publication Date: April 1932
Attached jpeg of the note on concentrating isopropanol.

I don't know but it looks like maybe the isopropanol could be used over and over again. Just remove water and use it again. Again probably not worth
the bother, but it might be worth saving the solvent and doing it in large batches.
I suppose since the isopropanol is used in such large proportion compared to what is used in the case of ethanol or methanol, the solvent could
probably just be recharged with reactants and used again.
[Edited on 2-4-2013 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
In an earlier post I was speculating that aluminum isopropoxide could be useful for freebasing hydrazine from dihydrazine sulfate, but thinking more
about this it seems like the use of that reagent could be problematic a couple of different ways. I don't know if this first possible complication
would occur but it does seem possible.
http://www.sciencemadness.org/talk/viewthread.php?tid=1128&a...
The possibility of a complication presented by the insolubility in the isopropanol of any transitional Al(OH)3 which may be needed to function as the
reactive base, exists IMO and may prevent the formation of the aluminum sulfate from a direct reaction of the soluble alkoxide and the dihydrazine
sulfate. The aluminum isopropoxide would I think be fine however for drying the isopropanol and / or stripping the H2O content from an already
freebased hydrazine hydrate in solution in the isopropanol, and the insoluble Al(OH)3 byproduct could be filtered leaving the anhydrous hydrazine in
anhydrous isopropanol. This would probably be better targeted to a slightly less than anhydrous solution in order to avoid any excess of unreacted
aluminum isopropoxide which would then contaminate the azide wished to be produced later as only the sodium salt.
Bearing these two potential complications in mind, it points to sodium ethoxide as the better choice for such a scheme, probably even if it is to be
used as the substituted NaOH "base" dissolved in isopropanol, unless a sodium isopropoxide may be formed similarly and as easily as is the sodium
ethoxide. The sodium alcoholate is easier to make and requires no distillation.
Sodium ethoxide related reading here
http://www.sciencemadness.org/talk/viewthread.php?tid=17074&...
and here
http://www.sciencemadness.org/talk/viewthread.php?tid=2656
See US1978647 attached.
Continuing with the "brainstorming" on this the aluminum isopropoxide may be useful for preparation of the sodium isopropoxide, simply by reacting
with NaOH. Evidently the sodium isopropoxide is much more soluble in the isopropanol than is NaOH so this would address the low solubility issue for
NaOH in isopropanol. It is possible and likely the sodium isopropoxide could be used for the base in the nitrosation stage as well. Attached is a
product sheet showing sodium isoproxide sold as a 12% solution in isopropanol and what may be its additional solubility I don't know but it is at
least 12%.
http://www.sciencemadness.org/talk/viewthread.php?tid=13212&...
Attachment: US1978647 Acetone Precipitation of Sodium Ethoxide.pdf (201kB)
This file has been downloaded 892 times
Attachment: Sodium_Isopropoxide.pdf (74kB)
This file has been downloaded 1083 times
[Edited on 3-4-2013 by Rosco Bodine]
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Topped
3-4-2013 at 01:01 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A few more dots yet to connect about sodium azide
My earlier declaration about having fully connected the dots on the matter of sodium azide synthesis was a bit premature.
It seems there is some more yet to say about this.
Two patents in particular, US1628380 and US5208002 are especially relevant and were mentioned in my first anonymous e-mails to Polverone 10 years ago.
http://www.sciencemadness.org/talk/viewthread.php?tid=470&am...
The later patent represents an improvement over the earlier patent. In the 10 years interim neither patent has been attached until now. Hmmm.
Something useful has there been hidden in plain sight, or perhaps overlooked. Compare the two patents and the technical difficulty in particular, for
the anhydrous technique which resorts to even use of sodium ethoxide to maintain a low water content and facilitate a more complete reaction and
easier isolation of the product in some better yield, but the price paid for that being more difficult manipulations. Obviously the process is still
entirely workable even for the reaction system where water content is substantial. Here the more modern patent looks at the economics which arguably
show it is easier to simply increase the batch size and use less rigorous conditions to offset any lesser yield, and showing by example 3 that the
implementation of the simpler conditions is entirely workable on a lab scale.
US1628380 Azide from hydrazine and organic nitrite via low H2O content reaction system
Attachment: US1628380 Azide from hydrazine and organic nitrite via low H2O content reaction system.pdf (163kB)
This file has been downloaded 916 times
US5208002 Azide from organic nitrite and hydrazine via high H2O content reaction system
Attachment: US5208002 Azide from organic nitrite and hydrazine via high H2O content reaction system.pdf (159kB)
This file has been downloaded 830 times
So there really are 2 entirely valid approaches where the same chemistry applies but the manipulations are easier for the more modern method. It
would seem perfectly well adaptable to a dropwise addition of an organic nitrite, and should work with other alcohols as well, even though the
quantities may have to be adjusted because the model reaction of example 3 of the patent describes the work using methanol. Refinements would seem
possible beyond what the more modern patent describes. I will study this further, to estimate how most conveniently to approach similar reaction
system conditions as described for the later patent's less rigorous conditions, having easier manipulations involving the freebasing of hydrazine
tolerating a higher level of hydration which evidently poses no obstacle to producing a good yield, but only changes the manipulations involved in
isolation of the product, requiring some evaporation and concentration of the completed reaction mixture due to the product azide being in significant
amount but not completely dissolved in the completed reaction mixture due to the water content. Dealing with simply concentrating the reaction
mixture to crystallize out the dissolved product is a routine manipulation which presents no special difficulty.
http://www.youtube.com/watch?v=lJiznhUEc8g
<iframe sandbox width="640" height="480" src="http://www.youtube.com/embed/lJiznhUEc8g" frameborder="0" allowfullscreen></iframe>
[Edited on 4-4-2013 by Rosco Bodine]
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
The point of adding the NaOH in two portions, is that the first formation of the paste of NaHSO4, hydrazine hydrate and alcohol is that the hydroxide
does not need to dissolve in the alcohol (at least this is my intuition; I haven't really analysed the reaction to be certain).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
What is the reason why you think the acid sulfate of sodium is the initial neutralization byproduct? I don't believe that is correct. I believe the
byproduct is the normal sulfate ....start to finish. I am pretty certain the dihydrazine sulfate forms progressively as the first half of the base is
added by observation of the liquification of half the solids of the slurry, which seems to melt to a thinnest viscosity when fairly hot ~85C est. not
measured.
At the half neutralization this is what I think you get
2 NH2NH2-H2SO4 + 2 NaOH ---> (NH2NH2)2-H2SO4 + Na2SO4 + 2 H2O
solubility for monohydrazine sulfate 3.4 g per 100ml H2O @ 25C
solubility for dihydrazine sulfate 202.2 g per 100ml H2O @ 25C freezing point / m.p. 45-55 C
Then during the freebasing stage of the remaining base addition
(NH2NH2)2-H2SO4 + 2 NaOH ----> 2 NH2NH2-H2O + Na2SO4 + ( Na2SO4 + 2 H2O from above )
It was my thought that in the freebasing stage is where it may be an advantage to use 1 mole of Ca(OH)2 in place of the 2 moles of NaOH hoping to
form the double salt CaSO4-Na2SO4 via reaction with the Na2SO4 from the first reaction above. The double salt is anhydrous and would not pull water
of crystallization which here is mixed with hydrazine hydrate. It was my thought this "glauberite" double salt may be a more dense material and would
also reduce any impurity of sodium sulfate in the supernatant aqueous hydrazine hydrate....with or without alcohol. But I think the freebasing using
the calcium hydroxide would not be favored by much alcohol because of the low solubility of that base in alcohol and low enough solubility already in
H2O which would be worsened by alcohol. The alcohol would be added
after the freebasing is completed to take up the freebased hydrazine hydrate in water which will be the liquid phase of the "glauberite" slurry,
extracted using the alcohol swirled with the slurry, and decanted from the solids. I think this would probably work. If not, then we know straight
sodium hydroxide works with or without alcohol.
On that you are correct no alcohol is required for the neutralization of monohydrazine sulfate to begin with contact with solid NaOH, a single drop of
H2O will cause an exothermic reaction between the solids to initiate and it will continue from its own heat of reaction kicked further by the heat of
solution of the solid NaOH dissolving in the byproduct H2O. A large mass of the solids will get really hot really fast in the same way as does solid
NaOH react with sulfur and it will form a melted very hot slurry from the exotherm. The heating is so great it could be dangerous with really large
batches of several moles. Even with a 2 mole batch a portionwise neutralization approach is what I do because of the exotherm.
[Edited on 4-4-2013 by Rosco Bodine]
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I base my hypothesis on the supposition that the sodium ion essentially would replace the hydrazinium in the mono-hydrazinium sulfate. The reason that
I formulated this hypothesis to begin with was because I had observed a very marked difference between the mix at the two points (after the addition
of one equivalent and after addition of the second equivalent).
Your hypothesis would explain that as well. I haven't looked at equilibrium constants because, frankly, I was more interested in the fact
that it worked than why it worked (though if you wanted to explore the reaction further, the "why" does obviously
becomes important).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Actually I would amend what I said above about the normal sulfate forming from start to finish. What you are saying about the acid sulfate formation
could certainly be mathematically possible for the reaction from the very beginning of the base additions up to the point where 25% of the
neutralization occurs, which would be the midpoint of the first half reaction. It was just my first thought that it likely did not happen, but
looking at it further it would certainly be possible if the kinetics allow for it. I knew the dihydrazine sulfate formation was what was desired to
form and does form completely by the midpoint of the reaction, in either case. But theoretically in the first 25% of the overall neutralization the
math does work for the formation of an acid sulfate as a transient intermediate, also having 1 H2O byproduct at that point. In practical terms it
really makes no difference to the process by the time the midpoint has been reached, where the acid sulfate would have then been converted to the
normal sulfate before the freebasing could begin. but it is an interesting technical detail that the sulfate could initially be the acid sulfate
during the first 25% of the overall neutralization. What you are describing would be a two stage reaction leading to the midpoint, with the second
reaction below indicating the midpoint of the overall reaction.
2 NH2NH2-H2SO4 + NaOH ---> (NH2NH2)2-H2SO4 + NaHSO4 + 1 H2O ( midpoint of the first half-reaction )
(NH2NH2)2-H2SO4 + NaHSO4 + NaOH ----> (NH2NH2)2-H2SO4 + Na2SO4 + 1 H2O + ( 1 H2O from above )
( midpoint of overall reaction )
Thereafter as per shown earlier:
Then during the freebasing stage of the remaining base addition
(NH2NH2)2-H2SO4 + 2 NaOH ----> 2 NH2NH2-H2O + Na2SO4 + ( Na2SO4 + 2 H2O from above )
The simplified but really oversimplified summary equation which could be wriiten for the reaction overall would be
NH2NH2-H2SO4 + 2 NaOH ----> NH2NH2-H2O + Na2SO4 + H2O
Most people would just look at the summary equation and it does not accurately reflect the stepwise intermediate reactions which are occurring as
detailed above. The summary equation only shows the relative proportions of reactants overall, but is deceptive oversimplification about the reaction
mechanisms leading to that summary reaction which is not really showing the operative reactions as do actually occur.
It threw me a curve when you mentioned the formation of free hydrazine which certainly can only appear after the midpoint in the reaction, where by
that point there could not be any acid sulfate present. Some trace water I think is probably necessary to initiate the reaction which rate increases
as a result of its own byproduct water. The water allows dissolution of the solids which being solids are physically separated and isolated but
progressively dissolve and react in a local zone of solution that is the liquid phase provided by the small amount of water. Without any solvent, I
think the dry reactants would not quickly react at all because the subdivision of the materials is insufficient to bring the reactants into homogenous
contact, and the surface reaction limitation aspect would slow or possibly stop the reaction from continuing. The slurry of solid particles
dissolving into and byproducts precipitating from the solvent allows the reaction to proceed through the mass of reacting solids. And here the most
reaction enabling solvent is necessarily water at least in part, even when alcohol is also an additional solvent.
[Edited on 4-4-2013 by Rosco Bodine]
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Some trace water I think is probably necessary to initiate the reaction which rate increases as a result of its own byproduct water. The water allows
dissolution of the solids which being solids are physically separated and isolated but progressively dissolve and react in a local zone of solution
that is the liquid phase provided by the small amount of water.
|
Yes, this is what I meant, and also why it is not necessary or desirable to use a large amount of alcohol to dissolve the hydroxide. I believe that
the intimate contact between reactants in the slurry that forms until the midpoint is reached allows for a more complete (and faster) reaction than an
alcoholic solution of the hydroxide would.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Earlier in the thread S.C. Wack posted links for some German publications which may be helpful and I would ask for any German speakers to look at this
particularly the patent DE205683
I am curious about the use of ammonium sulfate mentioned in the patent.
If I understand the German patent describes distillation of the product as aqueous hydrazoic acid. Of course, while requiring special technique,
distillation would eliminate problems of isolation and purification of the intended product from an aqueous mixture where byproducts are in solution
or coprecipitated. Also there are reactions where the recreation of the free hydrazoic acid is done in situ using sodium azide and an acid for
further reaction such as with dicyandiamide to produce 5-aminotetrazole, so if this or ammonium azide as two examples were the ultimate intended end
product, the process may be adaptable for making use of the distillate directly, or to use its precursor mixture in some alternate scheme.
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
Curtius und Rissom Neue Untersuchungen uber Stickstoffwasserstoff N3H Journal fur praktische Chemie Oct. 1898 (attached)
Attachment: DE205683 Azide patent Stolle.pdf (109kB)
This file has been downloaded 1024 times
Attachment: Curtius und Rissom Journal fur praktische Chemie Oct. 1898.pdf (1.8MB)
This file has been downloaded 1341 times
[Edited on 12-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Hodgkinson patent related
In my first communication 10 years ago I mentioned the Hodgkinson patents, which have been posted about maybe 3 or 4 times since obtaining copies of
those patents. GB128014 is still intriguing. Hodgkinson indicates it is a fickle reaction occurring in a very specific narrow range of pH but is
somewhat cryptic about giving that pH a number.
http://www.sciencemadness.org/talk/viewthread.php?tid=874&am...
The reaction between an alkali nitrite and the neutral hydrazine salt is described to occur in an only slightly acidic solution not so acidic as to
redden litmus which would be a lower limit of 5 pH or perhaps 4.5 pH. Hodgkinson mentions the reaction as comparable to the pH of a solution of boric
acid but does not specify the strength of the example solution of boric acid, which even at about half of saturation at room temperature would be on
the threshold of being too low pH and would definitely redden litmus. So Hodgkinson is being mysterious while claiming yields of 85% for his
mysterious aqueous system process. There are recent patents claiming reaction of free hydrazine and an organic nitrite in an aqueous system in the
presence of a base.
And there are some early art references mentioning use of a inorganic nitrite in reaction with a hydrazine salt but the references describe acidic
conditions, some of them highly acidic conditions. There appear to be 2 different mechanisms and possibly 3 different mechanisms and the mechanism
involving a highly acidic reaction mixture is an oxidation, having about one third of the potential yields of the alternate scheme of nitrosation or
diazotization done in an alkaline reaction system. Hodgkinson seems to teach that the nitrosation mechanism will still occur if the reaction system
is only very slightly acidic, and does proceed in an aqueous reaction system. The pH range would seem to be in the range of bromocresol green or
bromocresol purple or alizarin. Hodgkinson offers no help on process monitoring indicators except for mention of litmus. Hopefully that can be
updated to an indicator having a sharp transition for process monitoring any experiments. What coloration may occur in the reaction mixture could be
a factor but is unknown.
Hodgkinson is cryptic also about the needed amount of alkali which he supposes must be present to maintain the precise acidity and specific pH for the
reaction system. Stoichiometry reveals that a full molar equivalent of NaOH or probably better NaHCO3 would be required along with the NaNO2 in order
for the reaction to remain close to neutral. But the description given by Hodgkinson states that the small impurity of alkali expected to be present
in the sodium nitrite would provide adequate buffering ....when the reaction stoichiometry does not suggest this would hold true. Hodgkinson hedges
his description by conceding that some added amount of alkali may be required if sufficient alkali is not present in the nitrite , when the
stoichiometry suggests that such need for additional alkali is a certainty. My own reckoning of what would be needed is an equivalent of NaOH or
NaHCO3, and probably what would be more workable still would be some replacement of that equivalent with a not yet predicted but probably small amount
of NaOAc which would serve as a buffer to maintain the highly specific pH requirement described by Hodgkinson.
This azide synthesis described by Hodgkinson continues to be intriguing to me, particularly because of the reported high yields from what appears to
be a mild reaction condition, but a fickle reaction requiring great precision to accomplish ...IF indeed it works as described or can be made to work
by some slight modification of what is described, after ascertaining what little secrets Hodgkinson may not have mentioned while describing the
process. I do not think it was just "inventors euphoria" or wishful thinking that would have Hodgkinson apply for 2 patents and associate the
invention with the Ordnance College of England, so there is probably some validity to the patent, even though it is clearly NOT a patent which makes
"full disclosure" of all the factual particulars in great detail. This leaves the matter art which must be puzzling to more persons than just myself,
with regards to ascertaining the full details of precisely how such a reaction must be conducted in order to work as described only generally. I have
tried in the past to make this process work and it failed, but I was not observant of the highly specific pH which evidently is THE absolutely
imperative requirement.
Studying the reaction and doing experiments possibly could lead to formulating exacting proportions of the required ingredients so that the target pH
would be achieved by
buffering and then the process would be reliable and reproducible.
Attached are some related reference materials which may help trying to model the reaction, particularly with regards to the proposed buffering aspect
for the nitrite in admixture with NaOH or more likely NaHCO3, and NaOAC which might be required to make the process workable. In place of NaOAC,
NH4OAc, borax, or ammonium borate might also be workable as part of the reasonably believed to be required buffering scheme which Hodgkinson neglected
to describe in full detail.
One thing of collateral interest which Hodgkinson did describe is the conversion of the slightly soluble hydrazine acid salt to the highly soluble
neutral salt. That much is something I and others have confirmed does occur.
Attachment: GB128014 Hodgkinson azide patent 1.pdf (185kB)
This file has been downloaded 968 times
Attachment: NO2 generation by NaNO2 plus Boric Acid solutions.pdf (81kB)
This file has been downloaded 1299 times
Attachment: pds-borates-optibor.pdf (88kB)
This file has been downloaded 1092 times
Attachment: pds-borates-ammoniumpentaborate.pdf (54kB)
This file has been downloaded 938 times
This would seem to be a likely candidate indicator
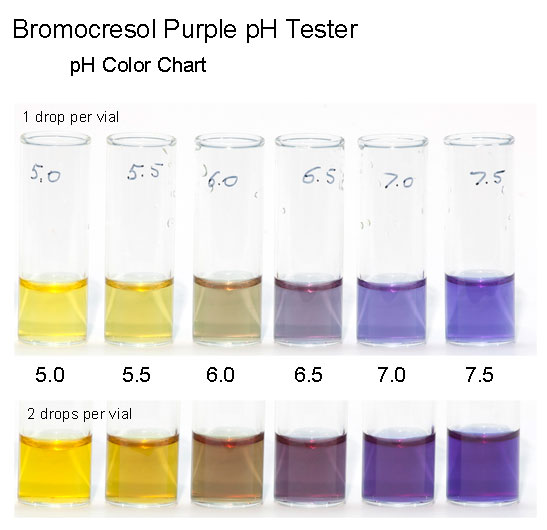
Further investigation of the Hodgkinson azide synthesis by experimental modeling and if possible by historical research into the process as conducted
looking at any industrial records or additonal writings of Hodgkinson or others employing the process, would make an excellent PhD dissertation and
thesis for some graduate student .... you know ...just sayin' this would be a cracker jack good one for some poor soul trying to think up something,
anything worthy as a subject for that important research paper.
Nothing like stirring the pot  Maybe this Hodgkinson azide synthesis will
intrigue somebody else as "lost art" which has far too long been missing from the literature and textbooks, seeing that odd omission should be
remedied with full disclosure which will remove it from obscurity as lost art, found once again. Now there's a doctoral thesis idea for ya. Maybe this Hodgkinson azide synthesis will
intrigue somebody else as "lost art" which has far too long been missing from the literature and textbooks, seeing that odd omission should be
remedied with full disclosure which will remove it from obscurity as lost art, found once again. Now there's a doctoral thesis idea for ya.
Biographical for Hodgkinson
William Richard Eaton Hodgkinson, Ph.D.
(1851–1935)
Professor of Chemistry and Physics, Ordnance College, Woolwich. Cotton Professor of Chemistry and Physics, R.M.A., Woolwich.
FYI & FWIW
http://parazite.nn.fi/roguesci/index.php/t-3211.html
(attached)
Attachment: Azides from Hydrazine Salts GB128014 and GB129152 [Archive] - The Explosives.pdf (28kB)
This file has been downloaded 1062 times
Also there was some discussion between myself and PHILOU Zrealone, ( Louis , Ph Z ) at the old alt.engr.explosives news group years ago regarding
uncertainties about the Hodgkinson patent which remains mysterious
http://alt.engr.explosives.narkive.com/hUjeaYs1/azides-from-...
(attached)
Attachment: Azides from Hydrazine Salts GB128014.pdf (265kB)
This file has been downloaded 856 times
[Edited on 13-4-2013 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
When I was discussing dissolving all the hydroxide in the alcohol I was talking about the reaction producing azide not freebasing.
N2H4 + CH3CH(NO2)CH3 + NaOH → NaN3 + CH3CH(OH)CH3 + 2H2O
or
N2H4 + CH3CH(NO2)CH3 + KOH → KN3 + CH3CH(OH)CH3 + 2H2O
I think I am right to dissolve all the hydroxide in the alcohol before adding the nitrite (right?).
Regarding freebasing I also use only a small amount of water (few drops) to start the freebasing. There definitely are two distinctly different
products produced during freebasing. IIRC viscous, translucent, gel like material is produced during the first half of the neutralization and then dry
crunchy white powder at the end. I always assumed the consensus on the forum was that it was first sodium bisulfate produced and then later sodium
sulfate. The mechanism where dihydrazine sulfate is the intermediate could be very useful, if that is what is actually happening.
If the Hodgkinson patent method does work then wouldn't it be a very desirable commercial process? Rosco, you haven't been able to make it work right?
I am not saying that it can't work, as I am not an expert, but from my limited experience there is a lot of bull or at the very least inaccuracies in
the patent literature.
[Edited on 16-4-2013 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Right. I couldn't make the Hodgkinson process work and I definitely tried using careful technique and different approaches and using pH test strips
frequently.
I think a variation on what Hodgkinson has described may possibly work but I have not figured it out yet. It is like a rubicks cube, I pick it up and
mentally play with it from time to time as a WTF is going on there kind of mental exercise  I don't like it when a patent stumps me. But some of them do, and this wouldn't be the first to come out of a "war college" laboratory,
there was another one called Emmansite IIRC which was actually just picric acid with an HNO3 of crystallization. Similar kinds of cryptic stuff is
sprinkled a few places with typographical errors in PATR that have caused perplexing misinterpretations, where the reader must become the proofreader
and editor to get the actual facts. I don't like it when a patent stumps me. But some of them do, and this wouldn't be the first to come out of a "war college" laboratory,
there was another one called Emmansite IIRC which was actually just picric acid with an HNO3 of crystallization. Similar kinds of cryptic stuff is
sprinkled a few places with typographical errors in PATR that have caused perplexing misinterpretations, where the reader must become the proofreader
and editor to get the actual facts.
Lately I have been reviewing some of the original and antique literature on azides and will be posting some of it here.
The method which I described for freebasing hydrazine hydrate into methanol, and the variation of Microtek for isopropanol are perfectly valid
syntheses for a lab scale sort of "one pot" method. The nearer anhydrous is the reaction system done in alcohol the better will be the yield and
simplifies things greatly since it can simply be filtered out of the spent reaction mixture. Towards that end of keeping the final water content
minimum I think the best approach is to use the base or the greatest portion of the base in the form of the alcoholate made separately. I believe
there may be a lower practical limit for the water content due to at least a tiny portion of water being needed in the freebasing of the hydrazine
sulfate.
One of the early researchers Thiele actually performed the reaction of hydrazine and ethyl nitrite using sodium methylate as the base and dehydrating
agent, both in excess with respect to hydrazine, and the reaction solvent being ether, Thiele obtained approximately quantitative yield based upon the
hydrazine. So this pretty well proves that for a one pot and single pass method, the "dry solvent" approach works for ether or alcohol.

The disadvantage of the aqueous system methods is needing to recycle unreacted materials, and having a heavy mixture of byproducts requiring some
scheme for separation, which presents isolation difficulties. Even for the aqueous system methods, in contrast with Hodgkinson other investigators
report that the organic nitrite ester and a basic reaction system provides a more rapid reaction and better yield than the alternative which
Hodgkinson describes incompletely, and that finding by others makes Hodgkinson even more doubtful.
I think the benefit of Hodgkinson is that some of the things suggested there may have validity ...and usefulness but in other context...so you can
make use of those parts, while thinking about the rest as dubious. Hodgkinson was getting on up in years ( 67 ) when the azide patents were
published, so there may be some sense of humor injected by the old man into those patents, the kind of humor that might arise with some senility  Old Ph.D's don't go nutters with age, they only become "eccentric" Old Ph.D's don't go nutters with age, they only become "eccentric" 
Much of the German is understandable from recognizing the chemistry, but any help with actual translation to English of these articles is welcome and
appreciated.
Zur Darstellung der Stickstoffwasserstoffsaure
Johannes Thiele
Berichte der deutschen chemischen Gesellschaft
Volume 41, Issue 2, pages 2681–2683, Mai–August 1908
(attached)
Attachment: Thiele Berichte.pdf (175kB)
This file has been downloaded 1280 times
Uber die Einwirkung von Salpetrigsaureestern auf Hydrazin, Phenylhydrazin und Benzhydrazid in alkalischer Losung
R. Stolle
Berichte der deutschen chemischen Gesellschaft
Volume 41, Issue 2, pages 2811–2813, Mai–August 1908
(attached)
Attachment: Stolle Berichte.pdf (161kB)
This file has been downloaded 921 times
Also here is an older article by Curtius which is more general about azides
Attachment: Curtius Journal_of_the_Switchmen_s_Union.pdf (691kB)
This file has been downloaded 762 times
[Edited on 17-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
DE205683 Translation to English
Thanks to DerAlte for translation assistance. I think this is pretty accurate but welcome any input from German speakers also understanding the
chemistry. The claims part is not included.
DE205683 Translation to English of Stolle azide patent
The reaction of nitrous acid with hydrazine gives hydrazoic acid in a known fashion. However, the yield of this compound is only small. It is now
found that HN3 is accessible from easily gotten Hydrazine hydrate or salts when one treats these compounds with nitrous acid ester, such as amyl
nitrite or ethyl nitrite, from nitrous acid and the alcohol, in alkaline solution (alcoholate, hydroxide, etc.) in appropriate solvents. Thus one
obtains up to 80% yield when one heats hydrazine hydrate in alcoholic solution with amyl nitrite and sodium methylate under reflux.
Otherwise it suffices to shake for a long time hydrazine sulphate with potassium hydroxide with added water, and adding to this solution some alcohol
and amyl nitrite. To isolate the resulting hydrazoic acid from the reaction products, the alcohol is distilled off , excess amyl nitrite and amyl
alcohol was blown off by steam and thereupon the alkaline solution distilled with appropriate amounts of ammonium sulphate and sulfuric acid. The
addition of ammonium sulphate avoids decomposition of HN3 by HNO2 (caused by amyl nitrite derived alkaline nitrite from its saponification, then freed
by sulfuric acid) and thus to prevent a loss.
Examples:
[1] Hydrazine hydrate 1kg was heated 25 hours with 4 kg amyl nitrite and a solution of 3 kg KOH in 15 kg alcohol the alcohol distilled off, the amyl
alcohol removed by steam distillation, the residue made up with 10 liters water solution and heated with about 3kg ammonium sulphate for 3 hrs, 1 kg
sulphuric acid added and distilled. Yield, 70-80% of theoretical (~640 g HN3)
[2] 1 kg hydrazine sulphate, 1 kg KOH, in 2 liters H2O were stirred for 5 hrs, with 4 liters alcohol 1 kg KOH and 1.5 kg amyl nitrite added and
stirred or shaken for 40 hours the alcohol distilled off, 0.75 kg ammonium sulphate added, the alcohol then steam distilled off, 5 liters H2O added,
and 0.5 kg sulphuric acid, and distilled. Yield 60-70% theoretical about 220 g HN3)
[3] 1 kg hydrazine sulphate 1 kg KOH 4 liters H2O stirred or shaken 5 hrs, 1 kg KOH and 2 kg amyl nitrite added, shaken or stirred for 100 hrs, the
amyl alcohol steam distilled off, residue heated 3 hrs with 0.75 kg ammonium sulphate, mixed with 5 liters H2O, and 0.5 kg H2SO4 added and distilled.
Yield 40-60% theoretical about 200 g HN3.
Attachment: Translation of DE205683 Stolle Azide patent.pdf (9kB)
This file has been downloaded 1756 times
[Edited on 17-4-2013 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Possible novel variation on Hodgkinson
The conditions which are described by Hodgkinson seem possibly adaptable and applicable to use of different reagents in a similar reaction scheme,
which I am uncertain will work. The variation which I have contemplated involves addition of isopropyl nitrite to an aqueous solution of acetone
azine, with the expectation that the organic nitrite will react with free hydrazine present from hydrolysis of the acetone azine to produce hydrazoic
acid, with a specific pH being maintained by concurrent addition of a base, perhaps sodium bicarbonate or ammonium bicarbonate or their respective
acetates in some combined buffer scheme. I believe that within a very specific and narrow range of pH that a similar reaction scheme as described by
Hodgkinson may occur and if workable would have advantages particularly in excluding unwanted byproducts.
Theory / Hypothesis / Experimental : When the dimethylketazine is dissolved in water, there is sufficient hydrolysis to cause the solution to become
basic due to the presence of free hydrazine as the hydrate with 2 molecules of byproduct ketone, in this case acetone, which results in a quaternary
system in hydrolytic equilibrium, consisting of hydrazine hydrate, water, and acetone, along with unhydrolyzed dimethylketazine. When isopropyl
nitrite is added to the alkaline system it should react with the hydrazine hydrate to form hydrazoic acid, and this will have the effect of lowering
the pH which favors further hydrolysis of the reserve lode of dimethylketazine, producing more hydrazine hydrate for further reaction with incoming
isopropyl nitrite. In the slightly alkaline pH range is where the reaction would be anticipated to proceed as described, where the hydrazine would be
present as the freebase hydrate, reactive with the isopropyl nitrite. The natural tendency of the reaction would be to become increasingly acid from
the hydrazoic acid, which would tend to interfere with the desired reaction, unless there is additional base added concurently along with the
isopropyl nitrite to neutralize the hydrazoic acid and maintain the slightly alkaline pH. If the pH were allowed to go too far acidic it would tend
to quench the desired reaction, because free hydrazine would itself act as a base to neutralize the hydrazoic acid forming hydrazine azide, and
another issue would arise with reactivity of the isopropyl nitrite with the acetone as in the acidic condition the hydrazine would compete to be
nitrosated, while nitrosation of the acetone would be favored in the acidic reaction mixture. Even though the reaction may be quenched by
insufficient neutralizing, it should recover and proceed when the pH is raised again into the operative range of alkalinity. The only foreseeable
issue for improper buffering is that some undesired byproduct may occur for the nitrosation of acetone which had not been avoided by allowing pH to
transiently range too low, but there would be no hydrazine loss and only a wastage of isopropyl nitrite as the consequence. The reaction should be
manageable using a color pH indicator dye, and pacing the additions of isopropyl nitrite and neutralizing base solution at a metered rate which keeps
the pH in the desired range of slightly alkaline. This is completely hypothetical, and has not been tested, nor can I find anything in the literature
for guidance. However, it seems that the natural hydrolysis of the dimethylketazine could be exploited as described to form an azide, as the sole non
volatile product, if the reaction proceeds in the correct pH range. If this hypothetical reaction proceeds with sufficient velocity is a big unknown,
and if it will work at all as anticipated is also unknown. It may work fine or not at all.
It seems like it would work. So that makes this hypothetical experiment intriguing, especially if it is novel, when it doesn't seem like somebody
else should not have thought of this possible? reaction before. I would have looked it up in the "Journal of Experiments that Seemed like they might
work but Failed" but I haven't been able to locate that huge compendium encyclopedic work anywhere. 
An interesting observation which I would make is that dimethylketazine could find usefulness as a sort of buffer in the orginal Hodgkinson reaction
scheme using a neutral hydrazine salt being gradually reacted with an alkali nitrite, because as that reaction becomes acidic it would tend to tie up
the hydrazine and quench the reaction, but the presence of dimethylketazine would tend to regulate that falling pH by additional hydrazine being freed
by the increasing acidity due to hydrolysis of the dimethylketazine. This suggests it may be possible that both the original Hodgkinson reaction
scheme, and the variation contemplated using dimethylketazine as the hydrazine source, might be possible to carried out in mixture as a system, where
the kinetics would favor the formation of azide.
For a possible color pH indicator I am thinking bromothymol blue would be about right

Thanks to gsd for the following article (attached)
STUDIES ON HYDRAZINE. THE HYDROLYSIS OF DIMETHYLKETAZINE AND THE EQUILIBRIUM BETWEEN HYDRAZINE AND ACETONE
E. C. Gilbert
J. Am. Chem. Soc., 1929, 51 (11), pp 3394–3409
DOI: 10.1021/ja01386a032
Publication Date: November 1929
Attachment: STUDIES ON HYDRAZINE.pdf (933kB)
This file has been downloaded 1049 times
[Edited on 21-4-2013 by Rosco Bodine]
|
|
|
| Pages:
1
..
8
9
10
11
12
..
18 |
|